What are the 4 pyrimidine bases?
You might be wondering why guanine is included in the list, as it’s typically associated with purines. That’s a great question! While guanine is a purine base, it’s also a component of DNA and RNA, and we need to consider its role in the larger context of nucleic acids.
Think of pyrimidine and purine bases as building blocks for DNA and RNA. Each nucleotide in these molecules is made up of a sugar molecule, a phosphate group, and one of these four bases. These bases pair up with each other in a specific way: adenine always pairs with thymine (in DNA) or uracil (in RNA), and guanine always pairs with cytosine.
This pairing is crucial for the structure and function of DNA and RNA. DNA stores our genetic information, and RNA helps to translate that information into proteins. These bases are like the letters in the genetic code, and their specific arrangement determines the sequence of amino acids in a protein.
Now, let’s look at the sequence GATCAATGC. We can identify two thymines (T) and two cytosines (C) in this sequence. So, the answer to your question is that there are four pyrimidine bases present in GATCAATGC.
Which of the following is a pyrimidine?
Cytosine, Uracil, and Thymine are all pyrimidines.
These are the building blocks of DNA and RNA, those essential molecules that hold the blueprint for life. Think of them like the letters in the alphabet of life.
Adenine and Guanine are the other crucial components of DNA and RNA, known as purines. These two groups of molecules pair up in a specific way, forming the iconic double helix structure of DNA.
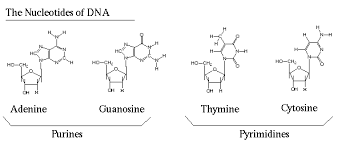
Pyrimidines have a single-ring structure, while purines have a double-ring structure. This difference in structure is essential for their specific pairing.
Cytosine pairs with guanine, and thymine pairs with adenine in DNA. In RNA, uracil replaces thymine and pairs with adenine.
Understanding these pairings is key to comprehending how DNA replicates and how RNA carries genetic information. It’s the foundation for understanding the intricate mechanisms of life!
What are 3 examples of pyrimidines?
Now, cytosine and thymine are found in DNA, while cytosine and uracil are found in RNA. This means that pyrimidines are essential components of our genetic material!
Here’s a little more about these pyrimidines:
Uracil is a pyrimidine base found in RNA. It pairs with adenine through hydrogen bonding, forming a crucial link in RNA’s structure.
Cytosine is another crucial pyrimidine base found in both DNA and RNA. It forms hydrogen bonds with guanine, contributing to the double helix structure of DNA and the various conformations of RNA.
Thymine, a pyrimidine base found only in DNA, pairs with adenine through hydrogen bonding. Thymine plays a vital role in DNA replication and repair, ensuring the integrity of our genetic code.
Pyrimidines are essential for life as we know it! They form the backbone of our genetic material, enabling the storage and transmission of our genetic information. They play a crucial role in protein synthesis, cell division, and a multitude of other biological processes.
Which is not a pyramid?
Let’s break down why a cylinder is a prism and not a pyramid.
A prism is a three-dimensional shape with two identical parallel faces, called bases, and other faces that are parallelograms. Think of a rectangular prism, like a shoebox. It has two rectangular bases connected by four rectangular faces. The bases are parallel and congruent (identical).
A pyramid is a three-dimensional shape with a polygonal base and triangular faces that meet at a point called the apex. Imagine a square pyramid, like the ones you often see in ancient Egypt. It has a square base and four triangular faces that meet at a single point above the base.
Now let’s look at the cylinder. A cylinder has two circular bases that are parallel and congruent. The sides of a cylinder are curved, connecting the two bases. Since it has two congruent parallel bases and curved side faces, it fits the definition of a prism, not a pyramid.
Remember, a pyramid has triangular faces that converge at a point, while a prism has parallelogram faces connecting its two congruent bases.
What are the 4 purine bases?
Let’s break down why these are called purines and what makes them special. Purines are one of the two types of nitrogenous bases found in nucleic acids, like DNA and RNA. They are characterized by their complex structure, featuring two rings. Adenine and guanine both have this characteristic two-ring structure, making them purines.
The other type of nitrogenous base is called a pyrimidine. Pyrimidines only have a single ring in their structure. In DNA, the pyrimidines are cytosine and thymine. In RNA, thymine is replaced with uracil.
So, to summarize:
Purines: Adenine and Guanine (present in both DNA and RNA)
Pyrimidines: Cytosine (present in both DNA and RNA), Thymine (present in DNA only), and Uracil (present in RNA only).
Think of purines and pyrimidines like puzzle pieces that fit together to form the structure of DNA and RNA. The specific pairing of purines and pyrimidines is crucial for the function of these nucleic acids. Adenine always pairs with thymine in DNA and uracil in RNA, while guanine always pairs with cytosine. This pairing is called complementary base pairing and is essential for DNA replication and protein synthesis.
What are pyrimidine bases?
You might be wondering, “What are pyrimidine bases?” They are essential building blocks of DNA and RNA, the molecules that hold the blueprints for life.
There are three main pyrimidine bases found in nucleic acids: thymine (T), cytosine (C), and uracil (U). These bases are slightly modified versions of the basic pyrimidine molecule, which has the formula C4H4N2.
Let’s break down these pyrimidine bases a little more:
Thymine (T) is found in DNA and pairs with adenine (A). It’s a crucial component of the genetic code, helping to store and transmit hereditary information.
Cytosine (C) is found in both DNA and RNA and pairs with guanine (G). Like thymine, it plays a vital role in the structure and function of DNA and RNA.
Uracil (U) is found only in RNA and pairs with adenine (A). It’s a key player in protein synthesis, helping to translate genetic information into the building blocks of proteins.
These pyrimidine bases are important because their specific arrangement within DNA and RNA determines the sequence of amino acids in proteins, which in turn dictates the structure and function of all living organisms.
Is T and C pyrimidine?
Nucleic acids are the building blocks of life, carrying the genetic information that makes us who we are. These complex molecules are made up of smaller units called nucleotides. Each nucleotide has three parts: a sugar, a phosphate group, and a nitrogenous base.
It’s the nitrogenous base that’s crucial for understanding pyrimidines. There are two main types of nitrogenous bases: purines and pyrimidines. Purines have a double-ring structure, while pyrimidines have a single-ring structure.
Now, back to our question: Is T and C pyrimidine? The answer is yes! Thymine (T) and Cytosine (C) are indeed pyrimidines. They are found in DNA (deoxyribonucleic acid), the molecule that holds our genetic code.
Uracil (U), another pyrimidine, takes the place of thymine in RNA (ribonucleic acid), which plays a vital role in protein synthesis.
Adenine (A) and Guanine (G), the other two important nitrogenous bases, are purines. They pair with pyrimidines to form the double helix structure of DNA, where A always pairs with T and G always pairs with C. This pairing is essential for ensuring the correct copying and transmission of genetic information.
Is uracil a pyrimidine?
Uracil plays a crucial role in RNA. It forms base pairs with adenine, similar to how thymine pairs with adenine in DNA (deoxyribonucleic acid). This pairing is essential for the structure and function of RNA. During DNA transcription, uracil replaces thymine, effectively creating a copy of the genetic information from DNA to RNA.
Let’s break down these key concepts:
Pyrimidines: These are a type of nitrogenous base with a single-ring structure. The other pyrimidine found in RNA and DNA is cytosine. Thymine, however, is found only in DNA.
Oxo groups: These are oxygen atoms bonded to carbon atoms in the pyrimidine ring. They play a significant role in the chemical properties of uracil and its ability to form base pairs.
Base pairs: These are formed through hydrogen bonds between complementary nitrogenous bases. Uracil forms two hydrogen bonds with adenine.
The fact that uracil is a pyrimidine is essential for understanding how RNA functions. It’s crucial for transcription and for the proper formation of RNA molecules.
See more here: Which Of The Following Is A Pyrimidine? | Which Of The Following Is Not A Pyrimidine
Which of the following is an example of a pyrimidine?
Cytosine and Thymine (C, T) are examples of pyrimidines. They are characterized by their single six-atom ring structure.
In contrast, Adenine and Guanine (A, G) are purines, which have a double-ring structure.
These bases form pairs in DNA – Adenine always pairs with Thymine (A-T) and Cytosine always pairs with Guanine (C-G). These pairs are called base pairs.
The A-T pair forms two hydrogen bonds, while the C-G pair forms three hydrogen bonds. These bonds hold the two strands of DNA together in a double helix. This structure is crucial for storing and transmitting genetic information.
Going Deeper into Pyrimidines:
Pyrimidines are an essential component of DNA and RNA. They are also found in other biologically important molecules, like coenzymes and vitamins.
Here’s a breakdown of some key features:
Structure: The core pyrimidine structure is a six-membered heterocyclic ring containing two nitrogen atoms at positions 1 and 3. This ring forms the basis for the various pyrimidine bases found in nucleic acids.
Variations: The pyrimidine bases found in DNA and RNA differ in their substituent groups attached to this core ring structure. These modifications play a crucial role in the base pairing interactions and the overall structure and function of nucleic acids.
Function: Pyrimidines are essential for:
DNA replication and repair: They are directly involved in the accurate copying of genetic information during cell division.
Gene expression: They are crucial for the process of translating genetic code into proteins, which carry out a wide variety of functions within the cell.
Medical Significance: Pyrimidines and their derivatives are important targets for drug development. For instance, some medications used to treat cancer work by interfering with pyrimidine metabolism, inhibiting tumor growth.
In essence, pyrimidines are fundamental building blocks of life, playing a crucial role in the storage, transmission, and expression of genetic information. Understanding their structure and function provides valuable insights into the intricate mechanisms of cellular processes.
Is guanine a pyrimidine nitrogen base?
Guanine is actually a purine base. Purines and pyrimidines are the two main types of nitrogenous bases found in DNA and RNA. They’re like the building blocks of these important molecules.
Here’s the breakdown:
Purines have a double-ring structure. The two most common purines are adenine (A) and guanine (G).
Pyrimidines have a single-ring structure. The most common pyrimidines are cytosine (C), thymine (T) in DNA, and uracil (U) in RNA.
So, to recap, guanine is a purine base, not a pyrimidine base.
Think of it this way: DNA and RNA are like long chains, and the nitrogenous bases are like the links in the chain. Adenine (A) always pairs with thymine (T) in DNA or uracil (U) in RNA, and guanine (G) always pairs with cytosine (C). This pairing is super important for the structure and function of DNA and RNA.
We can also understand this difference by looking at the chemical structures of purines and pyrimidines. Purines have a larger, more complex structure compared to pyrimidines. This structural difference is crucial for their interaction with each other and with the sugar-phosphate backbone of DNA and RNA.
Knowing the difference between purines and pyrimidines helps us understand how DNA and RNA are built and how they carry genetic information. It’s like having a codebook to decipher the language of life!
Why is pyrimidine more basic than pyridine?
Pyrimidine is a six-membered aromatic ring containing two nitrogen atoms. Pyridine also has a six-membered aromatic ring, but with only one nitrogen atom.
The key to understanding the difference in basicity lies in the nitrogen atoms’ ability to accept a proton and form a positive charge. Pyrimidine’s two nitrogen atoms can participate in resonance, which delocalizes the positive charge over the entire ring. This delocalization stabilizes the positive charge, making it easier for pyrimidine to accept a proton and thus more basic.
Pyridine, on the other hand, has only one nitrogen atom, and the resonance effect is less pronounced. This makes it less likely for pyridine to accept a proton and less basic compared to pyrimidine.
Another factor contributing to pyrimidine’s higher basicity is the presence of two sp2 hybridized lone pairs on its nitrogen atoms, compared to pyridine’s one. These lone pairs are readily available for protonation, enhancing pyrimidine’s ability to act as a base.
Think of it like this: Pyrimidine has two “hands” ready to grab a proton, while pyridine has only one. This makes pyrimidine more eager to accept a proton and thus, a stronger base.
In simple terms, pyrimidine is a stronger base than pyridine due to the following reasons:
Increased resonance: The additional nitrogen atom in pyrimidine allows for more extensive resonance, effectively distributing the positive charge and stabilizing the protonated form.
More lone pairs: Pyrimidine has two lone pairs on its nitrogen atoms readily available for protonation, enhancing its basicity.
Understanding the subtle interplay of these factors helps us appreciate the nuanced world of organic chemistry and why certain molecules possess distinct properties.
What are the two types of nitrogenous bases?
Purines are larger molecules with a double-ring structure. They consist of a six-membered ring fused to a five-membered ring. Think of them as the big guys in the nitrogenous base world!
Pyrimidines, on the other hand, are smaller molecules with a single six-membered ring structure. They’re like the petite, but powerful, members of the nitrogenous base family.
Now, let’s break down the specifics:
Purines include Adenine (A) and Guanine (G). These are crucial for forming the “rungs” of the DNA ladder. During metabolism, purines are broken down into uric acid, which is a waste product that is excreted in urine.
Pyrimidines include Cytosine (C), Thymine (T) (found only in DNA), and Uracil (U) (found only in RNA). These are also essential for building the DNA and RNA structures.
Let’s talk a bit more about Pyrimidine and its structure. While it’s similar to Pyridine (a nitrogen-containing aromatic compound), Pyrimidine has nitrogen atoms at positions 1 and 3 in the six-membered ring. This gives it specific properties that make it ideal for its role in DNA and RNA.
In summary, Purines and Pyrimidines are the fundamental building blocks of DNA and RNA. They play a vital role in storing and transmitting genetic information, ensuring life’s continuity!
See more new information: countrymusicstop.com
Which Of The Following Is Not A Pyrimidine: A Quick Quiz
Pyrimidines are nitrogenous bases that are essential building blocks of nucleic acids, like DNA and RNA. They are found in the nucleus of cells and play a critical role in genetic information.
There are three main pyrimidines: cytosine, thymine, and uracil. Cytosine is present in both DNA and RNA, while thymine is found only in DNA and uracil is found only in RNA.
Now, let’s dive into the question you’re really asking: Which of the following is not a pyrimidine?
Let’s look at some common options you might encounter:
Adenine: Adenine is a purine, not a pyrimidine.
Guanine: Guanine is another purine.
Thymine: Thymine is a pyrimidine.
Cytosine: Cytosine is a pyrimidine.
Uracil: Uracil is a pyrimidine.
Adenine: Adenine is a purine, not a pyrimidine.
Guanine: Guanine is a purine.
So, the answer is adenine and guanine. They are not pyrimidines; they are purines.
The Difference Between Purines and Pyrimidines
It’s important to understand the difference between purines and pyrimidines. Here’s a quick breakdown:
Purines have a double-ring structure consisting of a pyrimidine ring fused to an imidazole ring.
Pyrimidines have a single-ring structure.
Think of it like this: Purines are like a two-story house, while pyrimidines are like a single-story house.
The Importance of Pyrimidines
Pyrimidines are vital for life. They form base pairs with purines in DNA and RNA, and these base pairs are essential for:
Storing genetic information: Pyrimidines, along with purines, encode the instructions for building and maintaining an organism.
Replication of DNA: Pyrimidines play a key role in the duplication of DNA, ensuring that genetic information is passed on to new cells.
Protein synthesis: Pyrimidines are involved in the process of translating genetic information into proteins, which perform a wide variety of functions in the body.
What Happens When Pyrimidine Synthesis Goes Wrong?
When pyrimidine synthesis is disrupted, it can lead to a range of health problems, including:
Cancer: Mutations in genes involved in pyrimidine metabolism can contribute to the development of cancer.
Immune deficiencies: Pyrimidine synthesis is essential for the proper functioning of the immune system, so deficiencies can weaken the body’s defenses.
Neurological disorders: Pyrimidine metabolism is also linked to the health of the nervous system, and disruptions can lead to neurological problems.
Pyrimidine Metabolism: A Complex Process
The synthesis of pyrimidines is a complex process that involves multiple steps and enzymes. The key intermediates in pyrimidine biosynthesis are:
Carbamoyl phosphate: This molecule is formed from carbon dioxide, ammonia, and ATP.
Aspartate: This amino acid provides the nitrogen atom for the pyrimidine ring.
Orotic acid: This molecule is a precursor to both uridine and cytidine, which are the nucleoside forms of uracil and cytosine, respectively.
Pyrimidines: A Fundamental Building Block of Life
In conclusion, pyrimidines are crucial for life, playing a vital role in the storage, replication, and translation of genetic information. Understanding pyrimidine synthesis and metabolism is essential for comprehending a wide range of biological processes.
Now, let’s answer some frequently asked questions about pyrimidines:
FAQs
1. What are the differences between DNA and RNA?
DNA and RNA are both nucleic acids, but they have some key differences:
Sugar: DNA contains deoxyribose sugar, while RNA contains ribose sugar.
Bases: DNA uses thymine as one of its bases, while RNA uses uracil.
Structure: DNA is a double-stranded helix, while RNA is typically single-stranded.
2. How are pyrimidines synthesized in the body?
Pyrimidine synthesis occurs in the cytoplasm of cells. The process begins with the formation of carbamoyl phosphate, which is then combined with aspartate to form orotic acid. Orotic acid is then converted into uridine and cytidine, the nucleoside forms of uracil and cytosine, respectively.
3. What are some examples of drugs that target pyrimidine metabolism?
Several drugs target pyrimidine metabolism, including:
Methotrexate: This drug inhibits dihydrofolate reductase, an enzyme essential for the synthesis of tetrahydrofolic acid, which is a coenzyme required for pyrimidine biosynthesis. It is used to treat cancer and autoimmune diseases.
5-Fluorouracil: This drug is a pyrimidine analog that inhibits thymidylate synthase, an enzyme involved in the synthesis of thymine. It is used to treat cancer.
Leflunomide: This drug inhibits dihydroorotate dehydrogenase, an enzyme involved in the synthesis of orotic acid. It is used to treat rheumatoid arthritis.
4. Why are pyrimidine analogs used as anti-cancer drugs?
Pyrimidine analogs are often used as anti-cancer drugs because they can inhibit the growth and proliferation of cancer cells. These analogs compete with normal pyrimidines for incorporation into DNA, which can disrupt DNA replication and lead to cell death.
5. What is the role of pyrimidines in the immune system?
Pyrimidines are essential for the proper functioning of the immune system. For example, they are required for the synthesis of antibodies, which are proteins that help the body fight off infection.
6. What are some examples of diseases related to pyrimidine metabolism?
Disruptions in pyrimidine metabolism can lead to a variety of diseases, including:
Orotic aciduria: This is a rare genetic disorder that results in a deficiency of orotic acid phosphoribosyltransferase, an enzyme involved in the synthesis of uridine. This leads to the accumulation of orotic acid in the body, which can cause various symptoms, including anemia, growth retardation, and mental retardation.
Hereditary orotic aciduria: This condition affects the biosynthesis of pyrimidines.
Lesch-Nyhan syndrome: This is a rare genetic disorder that affects purine metabolism but can also affect pyrimidine metabolism, leading to the accumulation of uric acid.
Carbamoyl phosphate synthetase deficiency: This disorder affects the synthesis of carbamoyl phosphate, which is a crucial precursor for pyrimidine synthesis.
Dihydroorotate dehydrogenase deficiency: This disorder affects the synthesis of orotic acid, which is a precursor for both uridine and cytidine.
This is just a brief overview of pyrimidines and their role in biology. Remember, it’s crucial to always consult with medical professionals for any health concerns or before making any decisions related to your health.
Which of the following is not a pyrimidine? – Toppr
Question. Which of the following is not a pyrimidine? Thymine. Uracil. Guanine. Cytosine. A. Thymine. B. Uracil. C. Guanine. D. Cytosine. Solution. Verified by Toppr. Nucleic Toppr
Which of the following is not a pyrimidine nitrogen base? – BYJU’S
Adenine and Guanine are the purine nitrogen bases; Cytosine and Thymine (Uracil in case of RNA) are pyrimidine bases. BYJU’S
Which of the following is not a pyrimidine? – Toppr
Which of the following is not a pyrimidine? A. Thymine. B. Uracil. C. Guanine. D. Cytosine. Easy. Solution. Verified by Toppr. Correct option is C) Nucleic acids contain two types of Toppr
Which of the following is not a pyrimidine? (a) Thymine (b) Uracil
FREE SOLUTION: Problem 17 Which of the following is not a pyrimidine? (a) Th… step by step explanations answered by teachers Vaia Original! vaia.com
Which is not a pyrimidine? – doubtnut.com
Which is not a pyrimidine? A. Guanine. B. Thymine. C. Uracil. D. Cytosine. Video Solution. Text Solution. Verified by Experts. The correct Answer is: A. (A) Guanine doubtnut.com
Which of the following is not a pyrimidine? – Infinity Learn
The correct answer is There are two types of nitrogenous bases – Purines (Adenine and Guanine), and Pyrimidines (Cytosine, Uracil and Thymine). Infinity Learn
Which one of the following is not a pyrimidine base
Pyrimidine is an aromatic heteroyulp organic compound which is very similar to pyridine. The ring has nitrogen atoms at position 1 and 3 . There are three derivatives of Zigyan
Which of the following is not a pyrimidine – EMBIBE
(a) Thymine. (b) Uracil. (c) Guanine. (d) Cytosine. 100 % students answered this correctly. Check. Solution. Solve with us. Important Questions on Biomolecules. EASY. NEET. EMBIBE
Which one of the following is not a pyrimidine? – Infinity Learn
Which one of the following is not a pyrimidine? Easy. A. Cytosine. B. Uracil. C. Thymine. D. Guanine. Solution. Guanine is purine because it is dicyclic and heterocyclic nitrogen Infinity Learn
Which of the following is not a pyrimidine base? – Infinity Learn
The correct answer is Adenine and guanine are purine bases while cytosine, thymine and uracil are pyrimidine bases. Infinity Learn
Which Of The Following Is Not A Pyrimidine
Which Of The Following Is Not A Pyrimidine Base?
Which Of The Following Is Not A Pyrimidine Base? (A) Uracil (B) Gua…
Which Of The Following Is Not A Pyrimidine ?
Which Of The Following Is Not A Pyrimidine `N_(2)` Base-
Link to this article: which of the following is not a pyrimidine.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
