What happens when sulphur trioxide reacts with water?
When sulfur trioxide (SO3) reacts with water (H2O), it forms sulfuric acid (H2SO4). This is a classic example of a hydrolysis reaction.
Hydrolysis basically means “breaking down with water.” In this case, the water molecule helps break apart the bonds in sulfur trioxide, leading to the formation of sulfuric acid.
Sulfuric acid is a strong acid, meaning it readily releases hydrogen ions (H+) when dissolved in water. This makes it highly corrosive and a very useful chemical in many industrial processes.
Now, let’s get a little deeper into the reaction itself.
The reaction between sulfur trioxide and water is highly exothermic, meaning it releases a lot of heat. You can imagine it as a really energetic reaction! This heat release is one reason why sulfuric acid production is a carefully controlled industrial process.
The reaction is also very fast. Sulfur trioxide dissolves quickly in water, and the formation of sulfuric acid happens almost instantly.
Here’s a simplified way to represent the reaction:
SO3 + H2O → H2SO4
This reaction is really important in the industrial production of sulfuric acid. In fact, sulfuric acid is one of the most important industrial chemicals in the world! It’s used in so many different applications, from fertilizer production to battery manufacturing and even in the production of detergents.
It’s interesting to note that sulfur trioxide itself is a very reactive and corrosive gas. It’s not something you’d want to handle directly. But when it reacts with water, it forms the very useful sulfuric acid.
So, next time you hear about sulfuric acid, remember that it all starts with a simple reaction between sulfur trioxide and water!
Why is SO3 not dissolved in h2o?
Think of it this way: Imagine trying to mix oil and water. They don’t mix because they have different polarities. The same principle applies to SO3 and H2O.
While it’s true that the dissolution of SO3 in water is exothermic (meaning it releases heat), this isn’t the primary reason why SO3 doesn’t readily dissolve. The main reason is the difference in their polarities.
SO3 is a nonpolar molecule because the sulfur and oxygen atoms share electrons evenly. This means there’s no significant difference in charge across the molecule. H2O, on the other hand, is a polar molecule because the oxygen atom attracts electrons more strongly than the hydrogen atoms. This creates a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
Polar molecules tend to dissolve in other polar molecules because the positive and negative charges attract each other. Nonpolar molecules tend to dissolve in other nonpolar molecules.
Therefore, SO3 and H2O have difficulty dissolving in each other because of their different polarities.
What happens when SO3 is treated with water?
Let’s break it down:
SO3 is a covalent compound, meaning it’s made up of nonmetals sharing electrons. It’s actually a gas at room temperature.
Water (H2O) is a polar molecule, meaning it has a positive and negative end. This allows it to interact with the SO3 molecule, breaking it apart.
* The SO3 reacts with the water to form H2SO4, which is a very strong acid.
The reaction is so exothermic that the heat released can cause the water to boil, and the H2SO4 vaporizes, forming a dense white fog. This fog is actually a mixture of H2SO4 droplets and water vapor.
Here’s the chemical equation for the reaction:
SO3 + H2O → H2SO4
This reaction is a key step in the production of sulfuric acid, which is a very important industrial chemical. It’s used in many different industries, including the production of fertilizers, detergents, and batteries.
What is the product when sulfur trioxide is dissolved in water?
This reaction is highly exothermic, meaning it releases a lot of heat. The heat can even cause the water to boil, so it’s important to be careful when mixing these two substances. The reaction can be represented by the following chemical equation:
SO3(g) + H2O(l) → H2SO4(aq)
This reaction is essentially the addition of water to sulfur trioxide, forming sulfuric acid. Sulfuric acid is a strong acid, meaning it readily donates protons (H+) in solution. It’s widely used in various industrial processes, including the production of fertilizers, detergents, and batteries.
Here’s a closer look at the reaction:
Sulfur trioxide (SO3): This is a colorless gas with a pungent odor. It’s highly reactive and readily dissolves in water.
Water (H2O): Water acts as a reactant in this reaction, providing the hydrogen ions that will be incorporated into the sulfuric acid molecule.
Sulfuric acid (H2SO4): This is a strong acid, meaning it readily releases hydrogen ions in solution. It’s a corrosive liquid that’s essential to many industries.
The reaction is essentially the combination of sulfur trioxide and water molecules. The sulfur trioxide molecule readily accepts a water molecule, forming sulfuric acid. This process involves the breaking and formation of bonds between the atoms. The strong attraction between sulfur trioxide and water molecules makes this reaction highly favorable, leading to the rapid formation of sulfuric acid.
What is the balanced equation for sulphur trioxide reacts with water?
SO3 (g) + H2O (l) → H2SO4 (aq)
This equation shows that one molecule of sulfur trioxide (SO3) in the gaseous state (g) reacts with one molecule of water (H2O) in the liquid state (l) to produce one molecule of sulfuric acid (H2SO4) in the aqueous state (aq).
Let’s break down the reaction and its significance:
Sulfur trioxide (SO3) is a colorless gas with a pungent odor. It’s a highly reactive compound and a major contributor to acid rain.
Water (H2O) is a vital compound for life and plays a crucial role in many chemical reactions.
Sulfuric acid (H2SO4) is a strong acid widely used in various industries, including the production of fertilizers, detergents, and batteries.
The reaction between sulfur trioxide and water is an exothermic reaction, meaning it releases heat into the surroundings. This reaction is also a crucial step in the production of sulfuric acid, a vital industrial chemical. The reaction is highly favorable, meaning it proceeds readily to completion, effectively converting sulfur trioxide into sulfuric acid.
You might be wondering why this reaction is important. Well, sulfuric acid is a cornerstone of many industrial processes. It’s used in the production of fertilizers, which are essential for agriculture, and detergents, which are used in cleaning. It also plays a vital role in the production of batteries, which power our cars and other devices. However, the formation of sulfuric acid from sulfur trioxide is also a significant contributor to acid rain. When sulfur dioxide is released into the atmosphere, it reacts with oxygen to form sulfur trioxide. This sulfur trioxide then reacts with water in the atmosphere, forming sulfuric acid, which falls to the ground as acid rain. Acid rain can damage forests, lakes, and buildings, highlighting the importance of controlling sulfur dioxide emissions.
What happens to sulfur trioxide in rain water?
Now, this reaction has a significant impact on our environment. When sulfur trioxide is released into the atmosphere, it can travel long distances and eventually interact with water molecules in clouds. The reaction with rainwater creates sulfuric acid, which then falls back to the ground as acid rain. This acid rain can damage forests, lakes, and buildings, highlighting the crucial role sulfur trioxide plays in atmospheric chemistry and its effects on our planet.
Think about it this way: sulfur trioxide is a key player in the formation of acid rain. It readily reacts with water to form sulfuric acid, a powerful acid that can have harmful environmental effects. This reaction is a crucial part of understanding how sulfur emissions contribute to the acidity of rain.
See more here: What Happens When Sulphur Trioxide Reacts With Water? | Sulfur Trioxide And Water Equation
How does sulfur trioxide react with water?
When sulfur trioxide (SO3) and water (H2O) meet, they react to form sulfuric acid (H2SO4). This reaction is represented by the following chemical equation:
H2O(l) + SO3(g) → H2SO4(aq)
The (l) stands for liquid, (g) for gas, and (aq) indicates that the sulfuric acid is dissolved in water, forming an aqueous solution. This reaction is highly exothermic, meaning it releases a lot of heat. So much heat, in fact, that it can cause the surrounding water to boil and create a cloud of sulfuric acid vapor.
This reaction is not reversible, meaning that once the sulfuric acid is formed, it doesn’t easily break back down into sulfur trioxide and water. This makes the reaction highly efficient for producing sulfuric acid.
However, due to the extreme heat released, it’s important to be cautious when mixing sulfur trioxide and water. It’s best to carry out this reaction in a controlled environment, using specialized equipment and procedures. This ensures the process is safe and efficient, producing the desired amount of sulfuric acid without any unwanted side effects.
Think of it like this: It’s like mixing baking soda and vinegar. Both ingredients are safe on their own, but when combined, they react to create a bubbling, fizzing reaction. Similarly, sulfur trioxide and water react with a lot of energy, forming sulfuric acid.
If you’re interested in learning more about sulfuric acid and its uses, you can check out various resources online or in libraries. It’s a fascinating chemical with a wide range of applications, from making fertilizers to producing batteries. But remember, it’s important to handle it with care and only under the guidance of trained professionals.
What is the formula for sulfur trioxide?
But what’s sulfur trioxide actually used for? It’s a key ingredient in making two super important industrial chemicals: aryl sulfonic acids and sulfuric acid.
Let’s break down why sulfur trioxide is so important in these processes:
Aryl sulfonic acids: These acids are often used as detergents and in making dyes. Think of those colorful clothes you wear – sulfur trioxide played a role in making them possible!
Sulfuric acid: Now this is a big one. Sulfuric acid is one of the most widely used chemicals in the world. It’s used in making fertilizers, car batteries, and even in refining petroleum. It’s so versatile, it’s even used in making other chemicals.
So you see, although sulfur trioxide might seem like a simple compound, it has a big impact on the products we use every day.
How is sulfur trioxide used in a sulfonation reaction?
You might know that sulfur trioxide is used to make sulfuric acid, but did you know it also plays a key role in sulfonation reactions? These reactions are a bit like a chemical dance where an aromatic ring gets a new partner – a sulfonic acid group.
Let’s break it down. Sulfonation reactions are reversible reactions, meaning they can go both ways. They involve a process called electrophilic aromatic substitution, which is a fancy way of saying that an electrophile, like sulfur trioxide, attacks an aromatic ring.
So, how does sulfur trioxide get involved in this dance? Imagine the aromatic ring as a friendly molecule with a bunch of electrons. Sulfur trioxide, being an electrophile, is attracted to these electrons. It wants to share them! The sulfur trioxide attacks the aromatic ring, forming a new bond and creating a sulfonic acid group. This is like adding a new friend to the aromatic ring’s group!
Sulfonation reactions are super helpful because they allow us to create a wide range of useful compounds. The sulfonic acid groups can be further modified to create dyes, detergents, and even pharmaceuticals.
Now, let’s dive a little deeper into how sulfur trioxide does its magic. It’s not always used directly, sometimes it’s a little shy and prefers to work behind the scenes. You might see it in the form of oleum, which is a solution of sulfur trioxide dissolved in sulfuric acid. This is a very strong mixture and a good source of sulfur trioxide for sulfonation reactions.
Another way to use sulfur trioxide is in fuming sulfuric acid, which is basically sulfuric acid with a bit of sulfur trioxide dissolved in it. Both oleum and fuming sulfuric acid are powerful tools in the world of sulfonation reactions.
In the end, sulfur trioxide is a versatile player in the world of chemistry. It helps us create a wide range of useful compounds through sulfonation reactions, which are a bit like a chemical dance between aromatic rings and sulfonic acid groups. And just like a good dance, it’s all about finding the right partners and making things work!
See more new information: countrymusicstop.com
Sulfur Trioxide And Water Equation: Understanding The Reaction
Understanding the Reaction
The reaction between sulfur trioxide (SO3) and water (H2O) is a straightforward chemical reaction that produces sulfuric acid (H2SO4). Here’s the balanced chemical equation:
SO3 + H2O → H2SO4
This reaction is an example of a combination reaction, where two or more reactants combine to form a single product. This reaction is exothermic, meaning it releases heat into the surroundings.
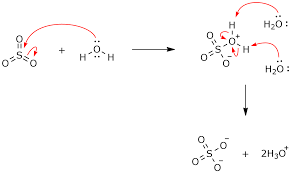
Mechanism of the Reaction
The reaction happens in two steps. First, sulfur trioxide reacts with a water molecule to form hydrogen sulfate (H2SO4). This intermediate compound is unstable and quickly reacts with another water molecule to form sulfuric acid.
Here’s the step-by-step breakdown:
1. SO3 + H2O → H2SO4 (Formation of Hydrogen Sulfate)
2. H2SO4 + H2O → H3O+ + HSO4– (Formation of Sulfuric Acid)
Importance of the Reaction
The reaction between sulfur trioxide and water is highly important because it’s the basis for the industrial production of sulfuric acid. Sulfuric acid is a crucial chemical used in a wide variety of industries, including:
Fertilizers:Sulfuric acid is used to produce phosphoric acid and ammonium sulfate, essential components of fertilizers.
Petroleum Refining:Sulfuric acid is used to remove impurities from crude oil during the refining process.
Metal Processing: Sulfuric acid is used in the production of metals such as copper, zinc, and lead.
Batteries: Sulfuric acid is the electrolyte in lead-acid batteries commonly used in cars.
Chemicals:Sulfuric acid is used in the production of a wide range of chemicals, including dyes, paints, and plastics.
Safety Precautions
Sulfuric acid is a highly corrosive and dangerous chemical. It can cause severe burns to the skin and eyes. It’s important to handle sulfuric acid with extreme caution and follow all safety guidelines.
Here are some important safety precautions to keep in mind:
Always wear protective gear: This includes gloves, goggles, and a lab coat.
Work in a well-ventilated area: Sulfuric acid releases sulfur dioxide, a toxic gas, when it reacts with water.
Avoid contact with skin and eyes: If you get sulfuric acid on your skin, immediately wash it off with plenty of water. If it gets in your eyes, flush them with water for at least 15 minutes and seek medical attention.
Store sulfuric acid safely: Sulfuric acid should be stored in a cool, dry, and well-ventilated area away from incompatible materials.
FAQs (Frequently Asked Questions)
1. What is the name of the reaction between sulfur trioxide and water?
The reaction between sulfur trioxide and water is called sulfuric acid synthesis.
2. Is the reaction between sulfur trioxide and water reversible?
The reaction is essentially irreversible under normal conditions. The formation of sulfuric acid is highly favored.
3. What is the role of sulfur trioxide in this reaction?
Sulfur trioxide acts as the acid anhydride in this reaction. It combines with water to form sulfuric acid.
4. What are the hazards of sulfuric acid?
Sulfuric acid is a highly corrosive acid that can cause severe burns to the skin and eyes. It can also release toxic fumes when it reacts with water.
5. How is sulfur trioxide produced?
Sulfur trioxide is produced commercially through the contact process, which involves the catalytic oxidation of sulfur dioxide.
6. What are some applications of sulfuric acid?
Sulfuric acid has a wide variety of applications in different industries. It’s used in the production of fertilizers, the refining of petroleum, the processing of metals, and the production of batteries and chemicals.
7. Is sulfuric acid a strong acid?
Yes, sulfuric acid is a strong acid. It fully ionizes in water, meaning it releases all its hydrogen ions (H+) when dissolved in water.
8. How does the reaction between sulfur trioxide and water contribute to acid rain?
Sulfur dioxide (SO2) is released into the atmosphere primarily from the burning of fossil fuels. When sulfur dioxide comes into contact with water and oxygen, it is converted to sulfuric acid, which then falls to the ground as acid rain.
9. What are the environmental impacts of sulfuric acid?
The production and use of sulfuric acid can have significant environmental impacts. The release of sulfur dioxide during the production process can contribute to acid rain, which can damage forests, lakes, and other ecosystems.
10. What are some alternative methods for producing sulfuric acid?
There are some alternative methods for producing sulfuric acid, but the contact process remains the most widely used method due to its efficiency and cost-effectiveness. Some alternatives include using plasma technology or electrochemical methods to produce sulfuric acid.
This article gave you a more in-depth look at the reaction between sulfur trioxide and water. This reaction forms sulfuric acid, a highly important industrial chemical with a wide range of applications.
Remember to handle sulfuric acid with care due to its corrosive nature. Always follow safety procedures when working with this chemical.
SO3 + H2O = H2SO4 – Balanced Chemical Equation
Word Equation. Sulfur Trioxide + Water = Sulfuric Acid. SO3 + H2O = H2SO4 is a Synthesis reaction where one mole of Sulfur Trioxide [SO 3] and one mole of Water [H 2 O] combine to form one mole of Sulfuric Acid [H 2 SO 4] ChemicalAid
SO3- Sulphur Trioxide Structure, Molecular Mass,
SO3 + H2O → H2SO4. Sulphur trioxide reacts with a base of sodium hydroxide and forms sodium hydrogen phosphate. The chemical BYJU’S
The Contact Process – Chemistry LibreTexts
Step 3: Converting sulfur trioxide into sulfuric acid. This cannot be done by simply adding water to the sulfur trioxide; the reaction is so uncontrollable that it Chemistry LibreTexts
Acid-base Behavior of the Oxides – Chemistry LibreTexts
Sulfur Oxides. Two oxides are considered: sulfur dioxide, SO 2, and sulfur trioxide, SO 3. Sulfur dioxide: Sulfur dioxide is fairly soluble in water, reacting to give a Chemistry LibreTexts
Mechanism of sulfur trioxide reaction with water to
I’ve drawn a more correct mechanism for the reaction of dilute $\ce{SO3}$ with water in the liquid phase: $$\ce{SO3 (aq) + 3H2O (l) -> SO4^2- (aq) + 2H3O+ (aq)}$$ $\ce{SO3}$ is a strong electrophile, Chemistry Stack Exchange
Sulfuric acid and the contact process [GCSE Chemistry only]
S (l) + O 2 (g) → SO 2 (g) This is not a reversible reaction – (l) means liquid and (g) means gas. Sulfur dioxide should not be released into the atmosphere as it contributes to…. BBC
GCSE CHEMISTRY – How is Sulfur Trioxide made into … – GCSE
How is Sulfur trioxide made into Sulfuric Acid? Sulfur trioxide will dissolve in water to make sulfuric acid. This is the balanced chemical equation for the reaction of sulfur trioxide GCSE SCIENCE
Sulfur Trioxide | Formula, Reactions & Preparation
Table of Contents. Sulfur Trioxide. Formula for Sulfur Trioxide. Lesson Summary. Frequently Asked Questions. Where is Sulfur Trioxide found? Near industrial areas, in the atmosphere, and… Study.com
Kinetics of Sulfur Trioxide Reaction with Water Vapor to Form …
Here, we calculate rate constants for reactions of sulfur trioxide with two water molecules. We consider two mechanisms: the SO 3 ···H 2 O + H 2 O reaction and ACS Publications
Write the balanced chemical equations for the following reactions …
Step 1: Representation of the chemical reaction: When Sulphur trioxide SO 3 reacts with Water H 2 O, Sulphuric acid H 2 SO 4 is formed. S O 3 ( Sulphur trioxide) + H 2 O ( BYJU’S
Reacting Sulfur Trioxide And Water
Sulfur Trioxide And Water Make Sulfuric Acid
Oleum. Sulfur Trioxide So3. Chemical Reactions
Making Sulfur Trioxide
Adding Water To Acid
Link to this article: sulfur trioxide and water equation.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
