Is nitrate soluble in water?
Nitrates are generally soluble in water. This means they dissolve readily when mixed with water.
So, yes, nitrate is soluble in water.
Let’s dive a little deeper into why this happens. Nitrates, like sodium nitrate (NaNO₃) and potassium nitrate (KNO₃), form ionic compounds. These compounds have a positive and a negative charge, creating an attraction between the ions. Water molecules, with their polar nature, can surround and interact with these charged ions, effectively breaking apart the ionic bonds and allowing the nitrate to dissolve. This process is called hydration.
The solubility of nitrates can vary slightly depending on the specific metal ion involved. However, in general, nitrates are highly soluble in water. This high solubility is due to the strong attraction between the polar water molecules and the charged ions of the nitrate compound.
Is chcl3 soluble in water?
Let’s break down why chloroform is only slightly soluble in water. Water is a polar molecule, meaning it has a positive and negative end due to the uneven distribution of electrons. This polarity allows water molecules to form strong hydrogen bonds with each other.
Chloroform, on the other hand, is a nonpolar molecule. Its structure doesn’t have a significant separation of charge. Because of this difference in polarity, chloroform molecules don’t form strong interactions with water molecules. They prefer to interact with other chloroform molecules, leading to limited solubility in water.
Think of it like trying to mix oil and water. Oil is nonpolar, and it doesn’t readily mix with polar water. Similarly, chloroform’s nonpolar nature limits its ability to dissolve in water.
While chloroform might not be very soluble in water, it readily dissolves in organic solvents like diethyl ether and ethanol, which are also nonpolar. This is because the forces of attraction between chloroform and these solvents are stronger than those between chloroform and water.
Can potassium nitrate be soluble in water?
Potassium nitrate dissolves in water because the strong attraction between the water molecules and the potassium and nitrate ions overcomes the attraction between the ions in the potassium nitrate crystal. This process, called hydration, allows the potassium nitrate to break apart into its constituent ions, K+ and NO3–, which then become surrounded by water molecules.
The solubility of potassium nitrate in water is affected by temperature. As the temperature increases, the solubility of potassium nitrate also increases. This means that you can dissolve more potassium nitrate in hot water than in cold water.
Potassium nitrate has a wide range of applications. It’s used as a fertilizer to provide nitrogen to plants, as an oxidizer in fireworks and explosives, and as a food preservative. It’s also used in the production of glass, ceramics, and matches.
Because of its solubility, potassium nitrate can easily be dissolved in water to create solutions for various applications. This solubility makes it a versatile compound used in many different industries.
What effect does KNO3 have on solubility?
KNO3’s solubility increases with temperature. This is because dissolving KNO3 is an endothermic process, meaning it absorbs heat from its surroundings. Think of it like a sponge soaking up water – the warmer the water, the more the sponge absorbs. Similarly, when you increase the temperature, more heat is available for KNO3 to absorb, leading to increased dissolution.
To understand this better, imagine adding KNO3 crystals to a beaker of water. When the crystals dissolve, they absorb heat from the water, causing the water to cool slightly. This cooling effect is a telltale sign of an endothermic process. Now, if you heat up the water, you’re essentially providing more heat for the KNO3 to absorb. This allows more KNO3 crystals to dissolve, making the solution more concentrated.
The solubility of KNO3 is a crucial aspect in various applications. For example, in agriculture, KNO3 is used as a fertilizer, and its solubility influences how effectively plants can absorb nutrients. Similarly, in the production of fireworks, the solubility of KNO3 plays a vital role in determining the combustion rate and the intensity of the flame.
Let’s consider a simple experiment to see this in action. If you were to add KNO3 to a beaker of cold water, you’d notice that a relatively small amount dissolves. However, if you heated the same beaker of water, you’d observe a significant increase in the amount of KNO3 that dissolves. This is a clear demonstration of the direct relationship between temperature and the solubility of KNO3.
Why is CH3Cl insoluble in water?
You see, water is a polar molecule. This means it has a slightly positive side and a slightly negative side. This is because oxygen, being more electronegative, pulls the shared electrons closer to itself, making the oxygen end of the water molecule slightly negative and the hydrogen end slightly positive. This difference in charge allows water molecules to form strong hydrogen bonds with each other.
Now, chloromethane is a nonpolar molecule. The chlorine atom, even though it is electronegative, isn’t as electronegative as oxygen. This means that the difference in electronegativity between carbon and chlorine is not large enough to create a significant dipole moment in the molecule. Since it has a very small difference in charge, chloromethane doesn’t have the ability to form hydrogen bonds with water molecules.
Think of it like trying to mix oil and water. They just don’t get along! The same principle applies here. Since chloromethane can’t form hydrogen bonds with water, it can’t dissolve in it.
To further illustrate, let’s imagine a dance party. Water molecules, being polar, love to dance with each other, forming strong hydrogen bonds. They are like the popular kids who only want to hang out with their own kind. Chloromethane, on the other hand, doesn’t have the right moves to join in the water party. It lacks the ability to form strong hydrogen bonds, so it’s left out in the cold, unable to mix. This explains why chloromethane is insoluble in water.
In essence, chloromethane doesn’t have the necessary properties to form hydrogen bonds with water molecules, making it incompatible and unable to dissolve.
Is toluene soluble in water?
Toluene is a common organic solvent that’s used in many industrial applications. You might be wondering if it’s soluble in water, and the answer is no, toluene is not very soluble in water. It’s considered poorly soluble because it mixes with water only in small amounts. This is because toluene is a nonpolar molecule, while water is a polar molecule.
Think of it like this: oil and water don’t mix, right? Well, toluene is similar to oil in that it doesn’t readily mix with water. However, toluene is soluble in many organic solvents, which are liquids that are also nonpolar. This means that toluene can dissolve in other compounds that have similar properties to itself.
Here’s a little more about why toluene and water don’t mix:
Polarity: Water molecules have a positive and negative end due to the uneven distribution of electrons. This polarity allows water molecules to form strong hydrogen bonds with each other. Toluene, on the other hand, has a more even distribution of electrons and is considered nonpolar. This means it doesn’t form strong hydrogen bonds with water molecules, which is why it doesn’t dissolve well.
Density: Toluene is less dense than water, meaning it’s lighter. Because of this, toluene will float on top of water.
Intermolecular Forces: Water molecules have strong intermolecular forces, known as hydrogen bonds, which hold them together. Toluene molecules, being nonpolar, have weaker intermolecular forces. This difference in forces contributes to the poor solubility of toluene in water.
Important Note: While toluene is not highly soluble in water, it’s still crucial to store it safely. It should be stored indoors in a designated flammable liquids room or cabinet, away from oxidizing materials. This is because toluene is flammable, and it’s important to handle it with care to prevent any potential hazards.
See more here: How Does Kno3 React In Water? | Is Potassium Nitrate Soluble In Water
What is the solubility of potassium nitrate in water?
Potassium nitrate is a compound that readily dissolves in water, forming a solution. This high solubility makes it a common ingredient in various applications. You might find it in fertilizers, explosives, and even food preservatives.
Now, let’s talk about the factors influencing its solubility.
Temperature plays a key role. As the temperature of water increases, the solubility of potassium nitrate also increases. This means that warmer water can dissolve more potassium nitrate than colder water. Think of it like adding sugar to your tea – the hotter the tea, the more sugar it can dissolve.
Concentration is another factor. If you already have a solution with a high concentration of potassium nitrate, it will be harder to dissolve more of the compound. This is similar to trying to cram more clothes into a suitcase that’s already packed – it gets harder and harder!
To illustrate this further, here’s an example: if you add a small amount of potassium nitrate to a cup of water, it will likely dissolve completely. However, if you try to add a huge amount of potassium nitrate to that same cup, you’ll reach a point where it can’t dissolve any more. This is known as the saturation point.
It’s important to note that the solubility of potassium nitrate in water can be affected by other factors, like the presence of impurities or the pH of the water. However, temperature and concentration are the most significant factors.
Understanding the solubility of potassium nitrate is crucial for various applications, from making fertilizers to creating fireworks. It helps us predict how much potassium nitrate will dissolve in a given amount of water under specific conditions.
Is sodium nitrate soluble or soluble?
The solubility of sodium nitrate, like many salts, increases as the temperature of the water rises. So, if you heat the water, you can dissolve even more sodium nitrate. This is because the increased temperature gives the water molecules more energy to break apart the sodium nitrate crystals and allow them to dissolve.
Let’s break down why sodium nitrate dissolves so well in water. Water is a polar molecule, meaning it has a slightly positive end and a slightly negative end. Sodium nitrate is also an ionic compound, meaning it’s made up of positively charged sodium ions (Na+) and negatively charged nitrate ions (NO3-). The positive end of water molecules is attracted to the negative nitrate ions, and the negative end of the water molecules is attracted to the positive sodium ions. These attractions allow the sodium nitrate to be surrounded by water molecules and dissolve in the water.
Does potassium nitrate dissolve in water?
Let’s dive a bit deeper into why potassium nitrate dissolves in water. The key is the interaction between the water molecules and the potassium nitrate molecules. Water is a polar molecule, meaning it has a slightly positive end and a slightly negative end. Potassium nitrate is an ionic compound, meaning it’s made up of positively charged potassium ions (K+) and negatively charged nitrate ions (NO3-).
When potassium nitrate is added to water, the water molecules surround the ions. The positive ends of the water molecules are attracted to the negative nitrate ions, and the negative ends of the water molecules are attracted to the positive potassium ions. This attraction is called hydration, and it weakens the bonds holding the potassium nitrate together. As a result, the potassium nitrate dissolves into its individual ions in the water.
There are a few factors that can influence the solubility of potassium nitrate. One factor is temperature. Generally, the solubility of salts, including potassium nitrate, increases with increasing temperature. This is because the increased kinetic energy of the water molecules helps to break the bonds holding the salt together. Another factor is the presence of other substances in the water. For example, the solubility of potassium nitrate can be reduced if there are other salts present in the water.
So, yes, potassium nitrate does dissolve in water! This is because of the interaction between the water molecules and the potassium nitrate ions, which leads to hydration and the dissolution of the salt.
What factors affect the solubility of potassium nitrate?
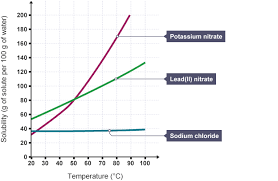
At a comfortable room temperature, you can dissolve about 31 grams of potassium nitrate in 100 milliliters of water. But, if you heat things up, the solubility of potassium nitrate gets even better! As the temperature goes up, more potassium nitrate can dissolve in the water.
Think of it this way: Imagine you’re trying to make a sugar-water solution. If the water is cold, the sugar dissolves slowly. But if you heat the water up, the sugar dissolves much faster. The same thing happens with potassium nitrate!
This change in solubility happens because of the energy involved. When you heat the water, you give the water molecules more energy. This energy allows them to break apart the potassium nitrate crystals and dissolve them more easily. It’s like giving the water molecules a little push to help them do their dissolving job!
So, if you’re working with potassium nitrate and need to control how much dissolves, temperature is your key factor. Just remember that as you heat things up, more potassium nitrate will happily join the party in your solution!
See more new information: countrymusicstop.com
Is Potassium Nitrate Soluble In Water: A Quick Guide
Hey there, chemistry enthusiasts! You’re probably wondering if potassium nitrate is soluble in water. The answer is a resounding yes!
In fact, potassium nitrate is quite soluble in water, especially at higher temperatures.
Let’s dive a little deeper and figure out why this is.
What Makes Something Soluble?
To understand why potassium nitrate dissolves in water, we need to think about the forces that hold things together.
Imagine the world of molecules, these tiny little building blocks. They’re always trying to hang out with other molecules they like. That’s why water molecules are attracted to each other, forming a hydrogen bond, a strong type of attraction.
Potassium nitrate, on the other hand, is made of potassium ions (K+) and nitrate ions (NO3-). These ions have a charge, which means they’re really attracted to other oppositely charged things.
So, what happens when you add potassium nitrate to water? The water molecules, with their partial charges, surround the potassium and nitrate ions, pulling them away from each other. The hydrogen bonds of water help break the ionic bonds in potassium nitrate, causing it to dissolve.
Think of it like this: The water molecules are like tiny little friends who are really good at getting potassium and nitrate ions to hang out with them, leaving their ionic bonds behind.
Factors Affecting Solubility
Now, there are some things that can affect how much potassium nitrate dissolves in water.
Temperature: The hotter the water, the more potassium nitrate it can dissolve. This is because the increased energy helps the water molecules break apart the potassium nitrate molecules more easily.
Pressure: While pressure doesn’t have as much impact as temperature, higher pressure can also increase the solubility of potassium nitrate.
Applications of Potassium Nitrate Solubility
The solubility of potassium nitrate in water has some really cool applications:
Fertilizers: Potassium nitrate is a common ingredient in fertilizers, providing plants with the nutrients they need to grow strong and healthy.
Fireworks: Potassium nitrate is a key ingredient in fireworks, helping to create the brilliant colors we love to see in the sky.
Food Preservation: Potassium nitrate can be used as a meat curing agent, helping to preserve and give meat a nice pink color.
Other Uses: Potassium nitrate has various applications, including in medicine, dentistry, and even as a component in some rocket propellants.
Interesting Fact:
Did you know that potassium nitrate is naturally found in some places like caves and deserts? It can form as a white, crystalline solid when evaporation occurs.
FAQs
Here are some frequently asked questions about potassium nitrate’s solubility:
1. Is potassium nitrate soluble in cold water?
* Yes, potassium nitrate is soluble in cold water, but the solubility is lower than in hot water.
2. What is the solubility of potassium nitrate in water at different temperatures?
* The solubility of potassium nitrate in water at different temperatures is as follows:
| Temperature (°C) | Solubility (g/100g H2O) |
|—|—|
| 0 | 13.3 |
| 20 | 31.6 |
| 40 | 63.9 |
| 60 | 110 |
| 80 | 169 |
| 100 | 246 |
3. Is potassium nitrate more soluble in water than sodium nitrate?
* Yes, potassium nitrate is more soluble in water than sodium nitrate.
4. How do I dissolve potassium nitrate in water?
* Simply add potassium nitrate to water and stir until it dissolves. You can use hot water to dissolve more potassium nitrate.
5. Is potassium nitrate a safe chemical?
* Potassium nitrate can be safe if handled properly, but it is important to wear appropriate safety equipment and to store it in a well-ventilated area.
In Conclusion
Potassium nitrate is a fascinating chemical with a variety of uses. Its high solubility in water makes it a versatile substance in many applications.
So, next time you see potassium nitrate, remember that it’s a substance that easily mixes with water, creating some pretty cool things!
Solubility Table of Compounds in Water at Temperature
Check the solubility of 128 common inorganic compounds (salts and acids) in water at different temperatures from 0 degrees Celsius to 100 C. Compound formulas are also MilliporeSigma
Solubility chart – Wikipedia
The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. 25 °C, 298.15 K). Wikipedia
Potassium Nitrate Solubility: A Comprehensive Guide
Potassium nitrate, KNO3, is a white to dirty gray crystalline solid that is highly soluble in water. The solubility of KNO3 in water has been extensively studied, and it techiescience.com
Potassium nitrate – Sciencemadness Wiki
Its solubility curve makes recrystallization easy, being only somewhat soluble in freezing water (13.3 g/100 ml at 0 °C), but very soluble in boiling water (246 g/100 ml at 100 °C). It is not hygroscopic, Sciencemadness Dot Org
Potassium nitrate | Definition, Formula, Uses, & Facts
These nitrates dissolve in rainwater, and white deposits of potassium nitrate are left behind when this water evaporates. Many caves throughout the world have large deposits of saltpetre due to large amounts of bat Britannica
Potassium Nitrate (KNO3) – Properties, Structure, Molecular
Potassium nitrate is a white solid soluble in water that is used in gunpowder, fertilisers, and toothpaste. Learn about its synthesis, structure, nutritional value, health hazards, and BYJU’S
How can I calculate solubility of potassium nitrate?
The solubility is about 90 g of KNO₃ per 100 g of water. Answer link. The only way to determine the solubility of potassium nitrate is to do an experiment or interpolate from a solubility graph. Solubility is Socratic
How soluble is potassium nitrate in water? – Vedantu
Ionic compounds are more soluble in water as compared to covalent compounds and potassium nitrate i.e., $KN{O_3}$ is an ionic salt of potassium ion \[({K^ Vedantu
potassium nitrate | Infoplease
Potassium nitrate is a chemical compound, KNO3, that is slightly soluble in cold water and very soluble in hot water. It is used in gunpowder, explosives, fireworks, matches, Infoplease
Solubility Of Kno3 Lab
Potassium Nitrate In Water
Potassium In Water (Reaction Only)
Solubility Of Potassium Nitrate Part 1 C0029
Is Kno3 Soluble Or Insoluble In Water?
Link to this article: is potassium nitrate soluble in water.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
