How to select pH of mobile phase in HPLC?
Organic Acids and Ionization
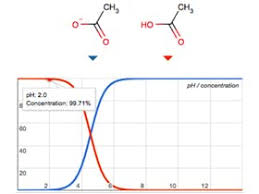
Many organic compounds are acidic, and their ionization state can significantly affect how they interact with the stationary phase of your HPLC column. By using a low pH mobile phase, we can minimize the ionization of these organic acids. This makes them more likely to interact with the non-polar stationary phase of the column, leading to better separation and sharper peaks. Imagine it like this: think of the non-polar stationary phase like a playground slide. If your compounds are neutral, they can easily slide down the column, separating from each other. But if they’re ionized, they’re like kids trying to climb up the slide – they get stuck and don’t move as easily.
Silanol Groups and Their Impact
Now, let’s talk about silanol groups, which are found on the surface of silica-based HPLC columns. These groups can be acidic and also ionize, potentially interacting with the compounds you’re trying to separate. When they do, it can lead to peak tailing and poor resolution. By adjusting the pH of the mobile phase to be lower, we can reduce the ionization of these silanol groups, minimizing their interference and giving you cleaner, more defined peaks.
Finding the Right Balance
It’s important to remember that the ideal pH for your mobile phase will depend on the specific compounds you’re analyzing and the characteristics of your column. While a pH range of 2-3 is often a good starting point, you may need to adjust it to achieve optimal separation. Remember that the goal is to minimize the ionization of your analytes while also keeping the silanol groups from interfering with your results.
How do you choose a buffer for chromatography?
The pH of your buffer should be optimized for your protein. Generally, you want to set the pH to be about 0.5 to 1 pH unit above or below the protein’s pI. This helps ensure your protein is ionized and soluble, making it easier to bind to the column.
Think of it this way: If the pH is too close to the protein’s pI, the protein will be mostly neutral and won’t interact much with the charged resin. By adjusting the pH, you can make the protein either more positively or negatively charged, depending on whether you want it to bind to a cation exchange or an anion exchange resin.
Buffer concentration is also important. A higher concentration can help to minimize changes in pH during the chromatography run. This is especially important if you’re dealing with large volumes of sample or if your protein is sensitive to changes in pH.
Finally, salt concentration plays a crucial role in the elution step. A higher salt concentration can help to compete with the protein for binding sites on the resin, leading to elution of the protein. The type of salt used is also important. For example, sodium chloride (NaCl) is a commonly used salt for elution, while others, like ammonium sulfate, can be used for initial sample preparation.
Choosing the right buffer conditions can seem complex, but it doesn’t have to be. By understanding the relationship between pH, buffer concentration, and salt concentration to your protein’s properties, you can set up successful ion exchange chromatography experiments. Don’t be afraid to experiment! Start with a basic buffer system and make adjustments to optimize for your specific protein and chromatography conditions.
What is the pH buffer for mobile phase?
Here’s the key principle: Select a buffer with a pKa value close to the desired pH of your mobile phase, ideally within +/-1 unit. Why is this so important?
Think of it like this: a buffer acts as a “pH stabilizer” for your mobile phase. When you choose a buffer with a pKa value near your target pH, the buffer effectively resists changes in pH, ensuring a stable environment for your separation. This is important because even slight changes in pH can significantly affect the retention of your analytes, leading to poor separation.
Let’s dive deeper into the importance of buffer selection in chromatography:
Analyte pKa: Each analyte has a specific pKa value, which represents its tendency to donate or accept protons (hydrogen ions) in a solution. The pKa value is directly related to the ionization state of the analyte at a particular pH. When the pH of the mobile phase is close to the analyte’s pKa, the analyte will exist in both ionized and non-ionized forms. This is where the buffer comes in. By carefully selecting a buffer with a pKa close to the mobile phase pH, you can ensure that your analyte remains primarily in its desired ionization state, leading to better separation and peak resolution.
Mass Spectrometry: If you’re using mass spectrometry as your detector, the choice of buffer becomes even more critical. Certain buffers can interfere with the ionization process in mass spectrometry, leading to poor signal-to-noise ratios or even complete suppression of your analyte’s signal. Therefore, you need to choose buffers that are compatible with your mass spectrometry system and won’t interfere with the ionization process.
Common Buffers: There are many commonly used buffers in chromatography, each with its own properties and applications. Some popular choices include phosphate buffers, acetate buffers, and Tris buffers. However, the best buffer for your specific application will depend on factors like the analyte’s pKa, the desired pH range, and the type of detector being used.
Remember, choosing the right buffer is an essential step in optimizing your chromatography experiment. By carefully considering the pKa values, compatibility with your detector, and the desired pH range, you can select the most appropriate buffer for achieving high-quality separation and reliable results.
What buffer solution is used in HPLC?
You’re probably wondering, what buffer solution is used in HPLC? Well, phosphoric acid and its sodium or potassium salts are the go-to buffer systems for reversed-phase HPLC. Why? Because they’re reliable and versatile. Think of them as the workhorses of the HPLC world.
But sometimes, you might need a different buffer solution. If you’re analyzing organophosphate compounds, you’ll want to swap out your phosphonate buffer for a sulfonate buffer. This is because phosphonate buffers can interfere with the analysis of these types of compounds.
Let’s break this down a bit more:
Buffers are essential for HPLC because they help to maintain a stable pH. This stability is crucial for achieving good separation and accurate results.
Reversed-phase HPLC is the most common type of HPLC, and it uses a non-polar stationary phase. This means that the analytes are separated based on their polarity.
Phosphoric acid is a strong acid, and its salts create a buffer system that can withstand a wide range of pH changes. This is why it’s a popular choice for HPLC applications.
Organophosphate compounds, such as pesticides, can react with phosphonate buffers, leading to inaccurate results. This is why you’ll often see sulfonate buffers used instead when analyzing these compounds.
Remember, selecting the right buffer solution for your HPLC analysis is crucial for getting reliable results. So, always consider the type of analytes you’re working with and choose the best buffer system to ensure a smooth and successful experiment.
How do I select a buffer for HPLC mobile phase?
Here’s a key rule of thumb for buffer selection: You should choose a buffer with a pKa value within plus or minus one pH unit of your desired mobile phase pH. Why? Because buffers work best within this range, resisting changes in pH. When the buffer’s pH equals its pKa, it has its maximum buffering capacity. This means it can effectively neutralize any small pH changes that might occur during the analysis.
Let’s break down this concept further:
pH: The pH of your mobile phase determines the ionization state of your analytes, directly impacting their interaction with the stationary phase and ultimately their retention times.
pKa: The pKa is a property of a specific buffer, indicating the pH at which the buffer is half-ionized. A buffer’s effectiveness is greatest when the pH of the mobile phase is close to its pKa.
To illustrate, imagine a buffer with a pKa of 7.0. If you aim for a mobile phase pH of 6.0, the buffer will be effective in resisting changes, keeping the pH close to 6.0. However, if your target pH is 4.0 or 8.0, the buffer’s ability to resist changes will be significantly weaker.
Choosing the right buffer is crucial for achieving reliable, reproducible results in your HPLC analysis. By understanding the relationship between pH, pKa, and buffer capacity, you can select a buffer that optimizes your separation and provides consistent and accurate data.
What makes a good mobile phase in HPLC?
The ratio of water to organic modifier can range from 0% to 100%. Increasing the amount of organic modifier helps elute the analytes from the column. Think of it like this: the organic modifier acts as a strong solvent, pulling the analytes off the stationary phase and into the mobile phase.
So, what makes a good mobile phase in reversed-phase HPLC? Here are some key things to consider:
Strength: The strength of the mobile phase refers to its ability to elute analytes from the column. A stronger mobile phase will elute analytes more quickly.
Selectivity: The selectivity of the mobile phase refers to its ability to separate different analytes. A highly selective mobile phase will separate analytes with different polarities or structures.
Compatibility: The mobile phase should be compatible with the detector and the stationary phase. It should not react with the analyte or the column.
Safety: The mobile phase should be safe to handle and dispose of.
By understanding these factors, you can choose the best mobile phase for your specific application. For example, if you are working with a complex mixture of analytes, you may want to use a gradient elution. This involves gradually increasing the concentration of the organic modifier over time, which helps to separate the analytes more effectively.
Remember, the key to a successful HPLC separation is a good mobile phase. So, take your time to choose the right one for your experiment, and you’ll be well on your way to achieving excellent results!
See more here: How Do You Choose A Buffer For Chromatography? | How To Select Buffer For Hplc Mobile Phase
What is a buffer in HPLC?
Think of a buffer as a guardian of that balance. It’s designed to resist changes in pH when small amounts of acid or base are introduced. This ensures a consistent environment for your separation, leading to more accurate and reliable results.
Buffers work best when used within a specific range, typically within one pH unit of their pKa (the pH at which the buffer is most effective). However, they can still provide decent buffering up to two pH units from their pKa.
There are lots of different buffers used in HPLC, and you’ll find a list of common ones in Table 1. The choice of buffer depends on your specific application and the compounds you’re separating.
For example, you might choose a phosphate buffer if you’re working with proteins because it’s known for its compatibility with biological molecules. Or, you might use a tris buffer if you’re working with nucleic acids.
Here’s a breakdown of the key considerations for choosing the right buffer in HPLC:
pH Stability: The buffer should maintain a stable pH throughout the analysis to avoid affecting the separation of your compounds.
Solubility: The buffer should be soluble in the mobile phase, otherwise, it can cause problems with the separation.
Compatibility: The buffer should not interfere with the detector used in the HPLC system.
Cost: Consider the cost of the buffer, especially if you’re working on a large scale.
By carefully selecting a buffer based on these factors, you can ensure optimal separation and accurate results in your HPLC experiments.
What is the function of pH buffer in reversed-phase HPLC?
Here’s why:
In reversed-phase HPLC, the mobile phase is usually a mixture of water and an organic solvent like acetonitrile or methanol. The stationary phase is a non-polar material like silica with a hydrophobic coating. The analytes, the compounds we’re trying to separate, are usually polar.
To separate analytes in reversed-phase HPLC, we adjust the mobile phase pH to a value significantly different from the pKa of the analytes. The pKa is the pH at which the analyte switches between its acidic and basic forms. By adjusting the mobile phase pH away from the pKa of the analytes, we ensure that the analytes are either fully protonated (acidic) or fully deprotonated (basic).
This is important because the retention of analytes, or how long they stay on the column, is heavily influenced by their ionization state. Retention is also dependent on the polarity of the analytes. If the mobile phase pH is near the pKa of the analyte, small changes in pH during the analysis can cause significant changes in the analyte’s retention, leading to poor separation.
Think of it like this: If the pH is near the pKa, it’s like having a see-saw where the analyte can easily switch between its acidic and basic form. This “see-sawing” makes it difficult to separate the analyte from other compounds.
By adjusting the mobile phase pH away from the pKa of the analyte, we create a stable system where the analyte remains either fully protonated or fully deprotonated. This stable state results in consistent retention and predictable separation of the analyte from other compounds.
This is why we use a pH buffer in reversed-phase HPLC, even though it doesn’t buffer directly in the typical pH range used. The buffer helps to stabilize the mobile phase pH, ensuring that even minor fluctuations in pH during the chromatographic process won’t affect the retention or selectivity of the analytes.
Can a buffer be substituted in HPLC?
Buffer substitution is a tricky subject. While it might seem tempting to simply substitute a different buffer, it’s not recommended. Here’s why:
Maintaining pH stability is critical: The pH of the buffer plays a crucial role in maintaining the stability of the analyte and the performance of the HPLC column. It impacts retention times, peak shape, and separation efficiency. Substituting a buffer can alter the pH and disrupt these factors, leading to inconsistent results.
Compatibility issues: Buffers are chosen based on their compatibility with the specific analytes, the column, and the mobile phase. Switching to a different buffer might cause unexpected interactions and lead to problems like peak tailing, poor resolution, and even damage to the column.
Calibration and validation: Your HPLC method is carefully calibrated and validated using a specific buffer. If you change the buffer, you’ll need to re-validate the method to ensure accuracy and reliability.
In essence, changing the buffer can be a domino effect that might lead to a whole chain of issues. The best practice is to use the specified buffer and replace it according to the recommended schedule. This minimizes the risk of unexpected variations and ensures consistent, reliable results.
What is the best buffer for HPLC?
For HPLC with UV detection, phosphate and acetate are the most popular choices because they have good buffering capacity and don’t interfere with UV detection. Phosphate is particularly versatile, offering a good range of buffering capacity from pH 5.8 to 8.0. Acetate buffers are ideal for a more acidic pH range, from pH 3.7 to 5.7.
Here’s a deeper dive into the properties of these two widely used buffers:
Phosphate buffers: Phosphate is a versatile buffer known for its good buffering capacity. It’s widely used in HPLC because it’s compatible with various mobile phases and doesn’t interfere with UV detection. Phosphate buffers are especially useful for separating analytes with a wide range of polarities. You can adjust the pH of phosphate buffers by mixing different proportions of monobasic potassium phosphate (KH2PO4) and dibasic potassium phosphate (K2HPO4).
Acetate buffers: Acetate is a good choice for more acidic mobile phases. It has good buffering capacity and is generally compatible with HPLC systems. Acetate buffers are particularly helpful for separating analytes that are sensitive to high pH levels.
When choosing a buffer, it’s also important to consider the specific requirements of your analysis. For example, if you are working with a compound that is sensitive to a particular pH, you may need to select a different buffer.
Remember, selecting the right buffer is just one part of optimizing your HPLC method. The choice of mobile phase, column, and flow rate also play critical roles in achieving good separation and analysis.
See more new information: countrymusicstop.com
How To Select Buffer For Hplc Mobile Phase: A Comprehensive Guide
Okay, so you’re diving into the world of HPLC, and you’re ready to start running some serious analyses. You’ve got your column, your detector, and your sample all set up, but you’re stuck on one crucial element – the mobile phase.
Specifically, you’re wondering how to select the perfect buffer for your mobile phase. And let me tell you, this is a decision that can make or break your experiment.
Why Buffers Are Essential
First, let’s talk about why buffers are so important in HPLC. They play a crucial role in maintaining the stability of your system and ensuring accurate results.
Here’s the lowdown:
pH control: Buffers help keep the pH of the mobile phase stable. This is critical for separating compounds that are sensitive to pH changes. You want to make sure your analytes are in their optimal ionization state for separation.
Chromatographic stability: Buffers can influence the stability of the stationary phase. The wrong buffer can lead to column degradation or unexpected interactions with your analyte.
Solubility: Buffers can improve the solubility of certain analytes. This can be especially helpful for compounds that are not very soluble in water.
Peak shape: A well-chosen buffer can improve the sharpness of your peaks, making your results easier to interpret.
The Buffer Selection Process
Now, let’s dive into the actual process of selecting a buffer for your HPLC mobile phase. It’s like a mini-adventure with some key steps to follow:
1. Know your Analyte: The first step is to understand the nature of your analyte. Are you working with a weak acid, a weak base, or a neutral compound?
Weak acids: You’ll likely need a buffer at a slightly acidic pH, allowing the compound to exist in its ionized form.
Weak bases: The opposite applies – a slightly basic pH will help these compounds ionize.
Neutral compounds: You can still use a buffer to help control the pH of your mobile phase, but it may not be as critical as it is for charged compounds.
2. Choose the Right Buffer System: Now comes the fun part – selecting your buffer system. Here are some popular options:
Phosphate buffers: These are widely used and have good buffering capacity in the pH range of 5.5 to 8.0. They are relatively non-toxic and compatible with most HPLC systems.
Acetate buffers: These are a great option for lower pH values (4.0 to 5.5) and are often used for separating acidic compounds.
Tris buffers: These buffers are good for slightly alkaline pH values (7.0 to 9.0). They are commonly used for protein separation.
Borate buffers: These are useful for separating carbohydrates and offer good buffering capacity in the range of 8.0 to 10.0.
Formate buffers: These can be used for acidic pH values, but they are less commonly used due to their potential to cause corrosion.
3. Consider the Column: Your column also plays a crucial role in buffer selection. Reverse-phase columns typically require mobile phases with organic solvents, while normal-phase columns often use polar solvents.
4. Think about your Detector: The detector you are using can also influence your buffer choice. Some detectors are sensitive to certain ions. For example, electrochemical detectors may be affected by the presence of certain buffer components.
5. Fine-tuning the Buffer Concentration: Once you’ve chosen your buffer system, you’ll need to determine the concentration. Here are some key points to keep in mind:
Buffering capacity: A higher concentration generally provides better buffering capacity, but it can also lead to increased background noise in your chromatogram.
Solubility: You need a buffer concentration that ensures your analytes are soluble.
Ionic strength: The ionic strength of the buffer can affect peak shape and retention time.
6. Test and Optimize: It’s important to test your chosen buffer system under your specific conditions. You can run a series of tests to optimize the buffer concentration, pH, and other parameters to get the best results for your analysis.
A Few More Tips for Buffer Selection
Use high-purity reagents: To avoid contamination and ensure accurate results, use high-purity reagents when preparing your buffers.
Filter your buffers: Filtering your buffers can remove any particulate matter that can clog your column or cause system problems.
Monitor the buffer performance: Regularly check the pH and conductivity of your buffer solution. It’s also a good idea to keep an eye on the pressure of your system. Changes in pressure can indicate issues with the buffer or the column.
FAQs:
Here are some of the common questions you might have about selecting buffers for HPLC mobile phases:
Q: What is a buffer and how does it work?
A: A buffer is a solution that resists changes in pH. It contains a weak acid and its conjugate base (or a weak base and its conjugate acid). When an acid or base is added to the buffer, the buffer components react to neutralize the added substance and maintain a relatively constant pH.
Q: Why is it important to use a buffer in the HPLC mobile phase?
A: Using a buffer in your mobile phase ensures that the pH of the system is controlled and stable. This is crucial for several reasons:
Optimizing analyte ionization: For proper separation, you want your analyte to be in the correct ionization state.
Maintaining column stability: Buffers help maintain the stability of the stationary phase, ensuring consistent separation over time.
Improving analyte solubility: Buffers can help solubilize certain analytes, making them easier to analyze.
Q: What factors should I consider when choosing a buffer system for my HPLC analysis?
A: When choosing a buffer system, consider the following:
Analyte characteristics: What is the analyte’s charge and how does it respond to pH changes?
Column type: Is it a reverse-phase or normal-phase column?
Detector type: Does the detector have any specific requirements or limitations?
Q: How can I optimize the buffer concentration and pH for my HPLC analysis?
A: Start by looking at the literature or online resources for guidance. Experiment with different buffer concentrations and pH values to see how they affect your separation. Monitor peak shape, retention time, and resolution to find the optimal settings.
Q: How can I prevent contamination or degradation of my buffer solution?
A: Here are some tips for preventing contamination and degradation:
Use high-purity reagents: Make sure to use reagents that are specifically designed for HPLC analysis.
Filter your buffer: Filter the buffer through a 0.45 µm filter to remove any particulate matter.
Store your buffer properly: Store your buffer in a clean, sealed container at a suitable temperature.
Monitor your buffer solution: Regularly check the pH and conductivity of your buffer to ensure it is still good to use.
Replace your buffer solution: Change your buffer solution regularly to prevent contamination and degradation.
Choosing the right buffer for your HPLC mobile phase is an essential step in obtaining accurate and reliable results. Take the time to understand your analyte, consider the column and detector, and optimize the buffer conditions. Remember, it’s a journey of discovery and experimentation, and it’s worth the effort to achieve high-quality separations!
HPLC Tips & Tricks: Mobile Phase Preparation Part 2 – Buffers
Learn the tips and tricks of selecting and preparing a suitable mobile phase buffer for your HPLC separations and method developments for samples that contain ionizable compounds. MilliporeSigma
A Guide to HPLC and LC-MS Buffer Selection
fruitful to adjust the mobile phase organic content (%B-solvent) to obtain acceptable retention for neutral and non-ionized compounds, then to adjust the pH to fine-tune HPLC
Buffers in Mobile Phase – Their Significance of Choosing
Buffer Selection. Buffer selection mainly depends on the desired pH of the mobile phase and which in turn depends upon the Phenomenex
HPLC Tips & Tricks: Mobile Phase Preparation – Buffers
Buffers prevent pH variations. Therefore, the proper buffer choice, in terms of buffering species, ionic strength, and pH, is the most critical step in HPLC method sepscience.com
Buffer choice for HPLC separations – Crawford Scientific
The mobile phase pH can change on standing, with ingress of CO 2 from the atmosphere for example, and a buffer can help to combat this effect to a certain extent. Similarly, volatile reagents, Element
Waters UPLC, UHPLC, and HPLC Column Selection and Mobile
Using a wide mobile-phase pH range is an effective approach to change compound selectivity. Increase selectivity for: – Acids (Peaks 3 and 6) – Bases (Peaks 1 and 4) Waters Corporation
HPLC Tips & Tricks – Mobile Phase Preparation – MilliporeSigma
Filtering removes particles from the prepared mobile phase and prevents clogging of the system and column. See more on mobile phase preparation in HPLC Tips & Tricks: MilliporeSigma
Selecting the Right Buffer – Fisher Sci
the buffer pK a value for good pH control of the mobile phase. Adequate buffer concentrations for HPLC tend to be in the 10-100 millimolar level depending on the size fishersci.com
Modern Trends and Best Practices in Mobile-Phase
Modern trends in LC mobile-phase selection and preparation include using simpler mobile phases, increased use of MS-compatible mobile phases, and eliminating filtration and certain mobile Chromatography Online
Basics Of Hplc_Part 1; Hplc Configuration/Mobile Phase/Buffer
A Guide For Selection Of Buffer For Hplc
The Use Of Mobile Phase Ph As A Method Development Tool
How To Choose The Correct Hplc Buffer?
Preparation Of A Mobile Phase Buffer (Ph 4.4) For Hplc Analysis.
Link to this article: how to select buffer for hplc mobile phase.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
