How many nodes are in a 3s orbital?
A 3s orbital has two radial nodes. We can determine this using a simple formula: (n – 1), where n represents the principal quantum number.
Since the 3s orbital has a principal quantum number of 3, we plug it into the formula: (3 – 1) = 2.
Now, let’s dive a bit deeper into what these nodes mean.
A node is a region in space where the probability of finding an electron is zero. Radial nodes are spherical surfaces where the probability of finding an electron is zero. These nodes are important because they help define the shape and energy of the orbital.
Think of it like this: imagine you have a balloon. The surface of the balloon represents the probability of finding an electron. Now, imagine you tie a string around the middle of the balloon. That string represents a radial node. The electron can’t be found on that string.
The number of radial nodes increases as the principal quantum number increases. This means that 4s orbitals have three radial nodes, 5s orbitals have four, and so on.
These nodes are a fundamental part of atomic orbitals and are essential for understanding the behavior of electrons in atoms.
How many nodal planes are in 3s orbital?
For an s orbital, *l* is always zero. This means that all s orbitals, including the 3s orbital, have no nodal planes.
Let’s break this down further.
Nodal planes are regions in space where the probability of finding an electron is zero. They are important because they help define the shape of an orbital.
A 3s orbital is a spherical orbital, meaning it has a shape that is symmetrical in all directions from the nucleus. It’s like a perfectly round ball. Because it’s symmetrical, there’s no point in space where the probability of finding an electron is zero.
Let’s think about it visually:
Imagine a 3D sphere. There are no planes slicing through this sphere that would define a region with zero probability of finding the electron. That’s why the 3s orbital has no nodal planes.
On the other hand, p orbitals, with *l* = 1, have one nodal plane. Think of a p orbital as a dumbbell shape. The nodal plane is the space in the middle of the dumbbell where there is zero probability of finding the electron.
Key takeaway:
The 3s orbital has no nodal planes because it is a spherical orbital with a symmetrical shape. The number of nodal planes is directly related to the azimuthal quantum number (*l*) of the orbital.
How many nodes are present in 3s and 3p orbital?
To understand this, we need to know a bit about quantum numbers. These numbers, like n, l, and ml, describe the properties of electrons within an atom.
n is the principal quantum number and indicates the energy level of the electron. It’s a whole number, and a higher n value means a higher energy level.
l is the azimuthal quantum number. It describes the shape of the orbital and has values ranging from 0 to (n-1). A l value of 0 corresponds to an s orbital, which is spherical. l = 1 corresponds to a p orbital, which has a dumbbell shape. And l = 2 corresponds to a d orbital, with more complex shapes.
Now, let’s talk about nodes. Nodes are regions in space where the probability of finding an electron is zero. They come in two types: radial nodes and angular nodes.
Radial nodes are spherical surfaces where the probability of finding an electron is zero. These occur between the nucleus and the electron cloud. The number of radial nodes is determined by the formula (n – l – 1).
Angular nodes are also known as planar nodes. These are flat planes where the probability of finding an electron is zero. The number of angular nodes is simply equal to the value of l.
So, for 3s orbitals, with n = 3 and l = 0, the number of radial nodes is (3 – 0 – 1) = 2. There are no angular nodes for s orbitals.
For 3p orbitals, with n = 3 and l = 1, the number of radial nodes is (3 – 1 – 1) = 1. There is one angular node for p orbitals.
In summary, the number of radial nodes in 3s, 3p, and 3d orbitals are 2, 1, and 0 respectively. This means that the 3s orbital has two spherical surfaces where the probability of finding an electron is zero, the 3p orbital has one spherical surface and one planar node, and the 3d orbital has no radial nodes and two angular nodes.
The concept of nodes is important in understanding the behavior of electrons within atoms. It helps us visualize the probability distribution of electrons in space and explains why certain orbitals have specific shapes and properties.
How many nodes are found in a 3s orbital quizlet?
You’re asking about the 3s orbital and how many nodes it has.
The 3s orbital has two radial nodes. A radial node is a region of space where the probability of finding an electron is zero.
The number of radial nodes in an orbital is related to the principal quantum number (n) and the angular momentum quantum number (l). For an s orbital, the angular momentum quantum number (l) is always 0.
The formula to calculate the number of radial nodes is:
n – l – 1
For the 3s orbital, n = 3 and l = 0. Plugging these values into the formula, we get:
3 – 0 – 1 = 2
Therefore, the 3s orbital has two radial nodes.
Let’s break down what this means:
1. Principal quantum number (n): This number determines the energy level of the electron. For example, n = 3 corresponds to the third energy level.
2. Angular momentum quantum number (l): This number determines the shape of the orbital.
– l = 0 corresponds to an s orbital (spherical shape)
– l = 1 corresponds to a p orbital (dumbbell shape)
– l = 2 corresponds to a d orbital (more complex shape)
– l = 3 corresponds to an f orbital (even more complex shape)
To summarize, the number of radial nodes in an orbital is determined by the principal quantum number (n) and the angular momentum quantum number (l). It represents the number of times the electron wave function changes sign in the radial direction. The 3s orbital has two radial nodes because it’s in the third energy level and has an l value of 0.
How to find the number of nodes in an orbital?
Total number of nodes = l + n – l – 1, which simplifies to n-1.
This means that the total number of nodes in an orbital is equal to the principal quantum number (n) minus 1.
Let’s break this down further.
Principal quantum number (n): This number describes the electron shell’s energy level. It can be any positive integer, with higher numbers indicating higher energy levels. For example, n = 1, 2, and 3 represent the first, second, and third electron shells, respectively.
Angular momentum quantum number (l): This number describes the shape of the orbital and has values ranging from 0 to n-1.
* l = 0 corresponds to an s orbital, which is spherical.
* l = 1 corresponds to a p orbital, which is dumbbell-shaped.
* l = 2 corresponds to a d orbital, which has a more complex shape.
* l = 3 corresponds to an f orbital, which has an even more complex shape.
Radial nodes: These are the points where the probability of finding an electron is zero. They occur within the orbital and are determined by the principal quantum number (n) and the angular momentum quantum number (l). The number of radial nodes is given by n – l – 1.
Angular nodes: These are the planes where the probability of finding an electron is zero. They are determined by the angular momentum quantum number (l). The number of angular nodes is equal to l.
Let’s consider an example:
A 2p orbital has a principal quantum number (n) of 2 and an angular momentum quantum number (l) of 1.
* The total number of nodes in a 2p orbital is n-1 = 2 – 1 = 1.
* This single node is an angular node, as l = 1.
You can calculate the total number of nodes for any orbital using this formula!
How many nodes are in 4p?
You’re right to think about radial nodes. These are areas where the probability of finding an electron is zero. For a p orbital, the number of radial nodes is always one less than the principal quantum number (n). Think of it like this: the higher the energy level (n), the more complex the orbital gets, and the more radial nodes it has.
So, for the 4p orbital, we have n = 4. Subtracting 2, as you mentioned, we get 2 radial nodes.
It’s helpful to visualize this. Imagine a 4p orbital as a dumbbell shape. Those two bulges represent areas where the electron is most likely to be found. Between the bulges, you’ll find a nodal plane – a flat surface where the probability of finding an electron drops to zero.
But there’s another kind of node – an angular node. These are surfaces where the probability of finding an electron is zero due to the shape of the orbital. For a p orbital, there’s always one angular node. This angular node is a plane that passes through the nucleus and divides the dumbbell shape into two lobes.
So, combining both types of nodes, the 4p orbital has a total of 3 nodes – 2 radial nodes and 1 angular node. This pattern applies to all p orbitals – the number of radial nodes increases with the principal quantum number (n), while the number of angular nodes always remains one.
See more here: How To Find Nodes In 3S? | How Many Nodes Are Found In A 3S Orbital
How many nodes are in a 3d orbital?
A node is a region in space where the probability of finding an electron is zero. Nodes are important because they help us understand the shape and energy of an atomic orbital.
The number of nodes in an orbital is related to the principal quantum number (n). Think of n as an energy level.
* For n = 1, there are no nodes in the 1s orbital.
* For n = 2, there is one node in the 2s and 2p orbitals.
* For n = 3, there are two nodes in the 3s, 3p, and 3d orbitals.
So, how many nodes are in a 3d orbital? The answer is two.
Here’s a deeper dive into the concept of nodes in 3d orbitals:
* Radial nodes: These nodes are spherical surfaces that occur at a certain distance from the nucleus. The number of radial nodes in an orbital is equal to n – l – 1, where l is the angular momentum quantum number, which determines the shape of the orbital. For a 3d orbital, l = 2, so there is one radial node.
* Angular nodes: These nodes are planes that pass through the nucleus. The number of angular nodes in an orbital is equal to l. For a 3d orbital, l = 2, so there are two angular nodes.
So, the 3d orbital has a total of two nodes – one radial node and two angular nodes. These nodes contribute to the complex, three-dimensional shape of the 3d orbital.
Let me know if you want to explore more about atomic orbitals or nodes! I’m here to help.
How many angular nodes does a 3s orbital have?
You’re right, the 3s, 3p, and 3d orbitals all have two nodes. But, there’s a catch. These nodes can be radial or angular, and they’re different!
The number of angular nodes is equal to the orbital angular momentum quantum number, l.
Here’s the breakdown:
l = 0 means there are no angular nodes (like the 1s or 2s orbitals).
l = 1 means there’s one angular node (like the 2p orbitals).
l = 2 means there are two angular nodes (like the 3d orbitals).
So, how many angular nodes does a 3s orbital have?
Since a 3s orbital has n=3 and l=0, it has zero angular nodes!
But, wait! What about those two nodes we mentioned? Well, those are radial nodes. These nodes occur when the probability of finding an electron at a certain distance from the nucleus is zero. Think of them like the “bumps” in the radial probability distribution, which show you where the electron spends the most time.
Here’s a way to visualize it:
Imagine you’re building a model of an atom. Instead of drawing a solid sphere around the nucleus, you’re using layers of yarn to represent the electron clouds. Each layer represents a different orbital.
* For the 1s orbital, you’d use a single layer of yarn. There are no nodes.
* For the 2s orbital, you’d add another layer of yarn, but with a gap in the middle. That gap represents a radial node.
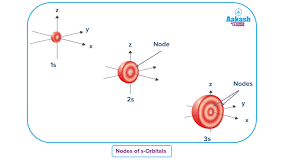
* For the 3s orbital, you’d add another layer of yarn, with two gaps. That’s two radial nodes.
The 3s orbital is kind of like a fluffy cloud with two “empty spaces” closer to the nucleus. These spaces are where the electron is less likely to be found!
How many radial nodes does a 3 s orbital have?
The 3s orbital has two radial nodes. This number is determined by the principal quantum number (n), which in this case is 3. The formula (n-1) tells us the number of radial nodes for an s orbital. So, for a 3s orbital, (3-1) = 2 radial nodes.
Imagine these nodes as regions within the orbital where the probability of finding an electron is zero. These regions are spherical in shape and occur as the electron’s wavefunction changes sign.
Think of it this way: The 3s orbital is like a sphere with two concentric layers where the electron is less likely to be found. These layers represent the radial nodes.
Let’s delve deeper to get a better understanding of radial nodes:
Radial nodes are regions in an atomic orbital where the probability of finding an electron is zero. They occur due to the wave-like nature of electrons and are different from angular nodes, which are regions where the probability of finding an electron is also zero but occur due to the angular momentum of the electron.
The 3s orbital has two radial nodes, which are spherical in shape and are located at specific distances from the nucleus. These nodes are important because they influence the shape of the orbital and its energy level.
The 3s orbital’s two radial nodes mean that the electron in this orbital has a higher energy than an electron in a 2s orbital, which has only one radial node. This is because the electron in the 3s orbital spends more time further away from the nucleus due to the presence of those two radial nodes.
Let me know if you have any more questions about orbitals and atomic structure!
How many nodes are there in the third electron shell?
For the third electron shell (n=3), there are twonodes in the 3s, 3p, and 3d orbitals. But what exactly are nodes?
In simple terms, a node is a region in an atomic orbital where the probability of finding an electron is zero. These regions are essentially areas of zero electron density. Think of them like “empty spaces” within an orbital.
Here’s the breakdown:
3s orbital: It has one spherical node.
3p orbitals: Each of the three 3p orbitals (3px, 3py, 3pz) has one planar node.
3d orbitals: Each of the five 3d orbitals has two nodes, which can be either spherical or planar, depending on the specific orbital.
The number of nodes in an orbital is directly related to the principal quantum number (n) of the shell. The general rule is that the number of nodes in an orbital is equal to (n – 1). So, for n=3, the number of nodes is (3-1) = 2.
Let’s visualize it:
Imagine a 3s orbital like a sphere with a hollow center. That hollow center is the node.
For a 3p orbital, picture a dumbbell-shaped structure with a flat plane cutting through the middle. That flat plane represents the node.
Understanding nodes is crucial for comprehending the behavior of electrons within atoms. They help explain various properties of atoms and molecules, such as their bonding characteristics and reactivity.
See more new information: countrymusicstop.com
How Many Nodes Are Found In A 3S Orbital?
Understanding Atomic Orbitals and Nodes
Before we jump into the 3s orbital, let’s quickly review what atomic orbitals are and what nodes are.
Imagine an atom as a miniature solar system. The nucleus, just like the sun, sits at the center. And the electrons, like planets, whiz around the nucleus in specific regions called atomic orbitals. These orbitals aren’t defined paths like planetary orbits. Instead, they’re more like fuzzy clouds where the probability of finding an electron is highest.
Now, nodes are specific regions within an orbital where the probability of finding an electron is zero. Think of it like a contour map of a mountain. The peaks represent the highest probability of finding an electron, while the valleys, where the lines cross, represent the nodes.
How Many Nodes are in a 3s Orbital?
The number of nodes in an atomic orbital is directly related to the principal quantum number (n) and the angular momentum quantum number (l).
Principal Quantum Number (n): This number tells us the energy level of the electron. Higher values of *n* mean higher energy levels. For the 3s orbital, *n* = 3.
Angular Momentum Quantum Number (l): This number describes the shape of the orbital and is related to the number of angular nodes. The value of *l* ranges from 0 to *n* – 1. For s orbitals, *l* = 0.
The total number of nodes in an orbital is given by *n* – 1. So, for the 3s orbital, we have:
Total number of nodes = n – 1 = 3 – 1 = 2
But here’s the catch: these nodes can be either radial nodes or angular nodes.
Radial Nodes: These nodes occur as spherical shells around the nucleus. They represent regions where the probability of finding an electron drops to zero due to a change in the electron’s radial distribution.
Angular Nodes: These nodes are planar and occur in specific directions. They represent regions where the probability of finding an electron is zero because the wavefunction changes sign.
Since the 3s orbital is a spherical orbital (*l* = 0), it has no angular nodes. This means that all the nodes in a 3s orbital are radial nodes.
Visualizing the 3s Orbital and its Nodes
Imagine a 3s orbital. At the center is the nucleus. The probability of finding an electron is highest near the nucleus. As you move away from the nucleus, you’ll encounter regions where the probability drops to zero. These are the radial nodes.
For the 3s orbital, there are two radial nodes. You can think of these as two concentric spheres around the nucleus where the electron density is zero.
In Summary
To recap, the 3s orbital has two nodes, both of which are radial nodes. There are no angular nodes in a 3s orbital because it’s a spherical orbital.
FAQs
Let’s tackle some common questions about 3s orbitals and nodes.
Q: What does it mean for an electron to have a zero probability of being found in a node?
A: It doesn’t mean the electron disappears or jumps over the node. It just means that at that specific point in space, the probability of finding the electron is zero. It’s like saying a specific point on a mountain has zero probability of being the highest point, even though the mountain itself exists.
Q: How do these nodes affect the chemical properties of an atom?
A: Nodes are crucial for understanding how atoms interact with each other. The shape of an orbital, which is determined by its nodes, influences the way atoms bond and form molecules. For example, the presence of nodes in the 3s orbital affects the size and energy of the electron cloud, which can impact the reactivity of the atom.
Q: Are there any other orbitals with similar node structures?
A: Yes, all s orbitals have only radial nodes. The number of radial nodes in an s orbital is always *n* – 1. So, a 1s orbital has no nodes, a 2s orbital has one node, a 4s orbital has three nodes, and so on.
Q: How do you determine the number of nodes in other orbitals, like p or d orbitals?
A: The number of nodes in any orbital is determined by *n* – 1. However, the difference is that for p, d, and higher orbitals, the nodes can be both radial and angular. For example, a 2p orbital has one node, which is an angular node, dividing the orbital into two lobes.
Q: How can I visualize these nodes in a more realistic way?
A: There are many online tools and software programs that can create 3D visualizations of orbitals, including their nodes. You can also find various diagrams and illustrations in textbooks and scientific journals that help you understand the concept visually.
Let’s Summarize
The 3s orbital has two nodes, both of which are radial nodes. The number of nodes in an orbital is determined by the principle quantum number (*n*) and the angular momentum quantum number (*l*). These nodes play a crucial role in determining the chemical properties of an atom.
Let me know if you have any more questions about nodes in atomic orbitals, or anything else related to chemistry!
How many nodes are present in 3s, 3p, and 3d orbitals? – BYJU’S
Rule for number of nodes: Nodes are always one less than the principal quantum number = n – 1. n = 1 in the first electron shell. Thus, there are no nodes in the 1s orbital. n = 2 in the second electron shell. There is only one node in the 2s and 2p orbitals. n = 3 in the BYJU’S
How many number of nodes present in 3s-orbital? – Toppr
Statement – 1: The number of radial nodes in 3s and 4p orbitals are not equal. Statement – 2: The number of radial nodes in 3s and 4p orbitals depend on ‘n′ and ‘l′ which are Toppr
Electronic Orbitals – Chemistry LibreTexts
The quantum number ℓ determines the number of angular nodes; there is 1 angular node, specifically on the xy plane because Chemistry LibreTexts
6.6: 3D Representation of Orbitals – Chemistry LibreTexts
All orbitals with values of \(n > 1\) and \(ell = 0\) contain one or more nodes. Orbitals with \(\ell = 1\) are p orbitals and contain a nodal plane that includes the nucleus, giving rise Chemistry LibreTexts
The Orbitron: 3s atomic orbitals – University of Sheffield
For any atom there is just one 3s orbital. The image on the top is deceptively simple as the interesting feature is buried within the orbital. That on the left is sliced in half to show that there the two spherical nodes of Mark Winter web site
The Orbitron: 3s atomic orbital radial distribution function
The 3s radial distribution function has two spherical nodes but the higher s orbitals have more. The number of nodes is related to the principal quantum number, n . In general, the ns orbital have ( n – 1) radial nodes. Mark Winter web site
Shells, subshells, and orbitals (video) | Khan Academy
An orbital is a space where a specific pair of electrons can be found. We classified the different Orbital into shells and sub shells to distinguish them more easily. This is also due to the Khan Academy
Atomic orbital – Wikipedia
Each orbital in an atom is characterized by a set of values of the three quantum numbers n, ℓ, and mℓ, which respectively correspond to the electron’s energy, its orbital angular momentum, and its orbital angular Wikipedia
Definition of Orbital Nodes – Chemistry Dictionary
Many real orbitals, such as 2s, 2p, and 3d orbitals have regions with both positive and negative phase. These regions are separated by nodes. For example, the diagrams below show 1s, 2s, and 3s orbitals. Notice how Chemicool
How many number of nodes present in 3s – orbital? – Toppr
How many number of nodes present in 3s-orbital? Medium. Solution. Verified by Toppr. Total number of nodes = n – 1. for 3s orbital value of n is 3 so total number of orbitals is Toppr
The Number Of Radial Nodes Of 3S And 2P Orbitals | Structure Of Atom Master Series | Quantum Numbers
How To Determine Number Of Angular Nodes, Radial Nodes, And Total Nodes Of Orbitals Examples
How Many Nodal Points Are Present In A 3S Orbital? | 11 | Structure Of Atom | Chemistry | Icse |…
How Many Number Of Nodes Present In 3S-Orital? Hint `: (N-1) =` Radial Nodes In S-Orbital
How Many Orbitals Are There In N=3, The 3Rd Energy Level Of An Atom?
Link to this article: how many nodes are found in a 3s orbital.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
