Let’s discuss the question: how many atoms are there in each of the following. We summarize all relevant answers in section Q&A of website Countrymusicstop.com in category: MMO. See more related questions in the comments below.

How do you find the number of atoms?
To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro’s number: 6.02 x 10^23.
How many atoms of each element there are?
An atom is an element. The two words are synonymous, so if you’re looking for the number of atoms in an element, the answer is always one, and only one.
How to find the Number of Atoms in a Molecule
Images related to the topicHow to find the Number of Atoms in a Molecule

How many atoms are there in?
According to the US Department of Energy’s Jefferson Lab, the answer is: 133,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000. That answer comes from an estimation of the number of atoms in each of Earth’s elements, like Iron, Oxygen, Silicon, Magnesium, Sulfur … etc.
How do you know how many atoms are in a sample?
So, if you are given the mass of an element, you use the periodic table to find its molar mass, and multiply the given mass by the reciprocal of the molar mass. This is Mass → Moles . Once you have moles, multiply by Avogadro’s number to calculate the number of atoms. This is Moles → Atoms .
How many atoms are in H2O?
Atoms join together to form molecules. A water molecule has three atoms: two hydrogen (H) atoms and one oxygen (O) atom. That’s why water is sometimes referred to as H2O.
Which has maximum number of atoms?
Thus, carbon has a maximum number of atoms.
How many atoms are in a period?
In Period 1 there are two elements, hydrogen (H) and helium (He). The second and third periods both contain eight elements, the fourth and fifth periods contain 18 elements, and the sixth and seventh periods contain 32 elements. Second, all of the elements are listed sequentially according to their atomic numbers.
How many types of atoms are there 2021?
There are more than 109 different types of atom – one for each element. Differences between the atoms give the elements their different chemical properties.
How many atoms are in bleach?
Bleach is made up of one sodium atom, one chlorine atom, and one oxygen atom. It has the chemical formula NaOCl, and the formal name is sodium…
How many atoms are in a cell?
Scientists estimate the average cell contains 100 trillion atoms. The number of atoms per cell is about the same as the number of cells in the body.
What are the 4 types of atoms?
- Description. Atoms are made of tiny particles called protons, neutrons and electrons. …
- Stable. Most atoms are stable. …
- Isotopes. Every atom is a chemical element, like hydrogen, iron or chlorine. …
- Radioactive. Some atoms have too many neutrons in the nucleus, which makes them unstable. …
- Ions. …
- Antimatter.
How many atoms are there in the atmosphere?
That is, we multiply the molar mass of each gas by its percentage of the mixture. Thus, the relative molar mass of air is 28.964. Rather than show the calculations for the other gases, I will summarize the results in a table. This 1 g of air contains 4.140×1022 atoms.
Calculate the number of atoms in each of the following: i) 52mol of Ar ii) 52u of He iii) 52g of He
Images related to the topicCalculate the number of atoms in each of the following: i) 52mol of Ar ii) 52u of He iii) 52g of He

How many elements are there?
The elements of the periodic table sorted by atomic number. click on any elements name for further chemical properties, environmental data or health effects. This list contains the 118 elements of chemistry.
How many atoms are in ag?
It means that if I have such a mass of silver, there are Avogadro’s number, NA=6.022×1023 , individual silver atoms.
How much atoms are in a mole?
The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 12.00 g C-12 = 1 mol C-12 atoms = 6.022 × 1023 atoms • The number of particles in 1 mole is called Avogadro’s Number (6.0221421 x 1023).
How many atoms are there in na2so4?
Answer and Explanation: In one molecule of sodium sulfate, or Na2 SO4, there are seven atoms in all. The chemical formula for this molecule tells us this.
How many atoms are in 2 waters?
There are three atoms in a water molecule: an oxygen atom and two atoms of hydrogen, which are bonded together like small magnets. The number of atoms present within each molecule represents the second conversion factor. 4 hydrogen atoms and 2 oxygen atoms are found in two water molecules.
How many atoms are there in caco3?
1 Expert Answer
In this compound there are 5 atoms. and by adding the subscripts together you get a total of 5 atoms.
What is the mass of 1 atom of oxygen?
The mass of an oxygen atom = 16 amu.
Which one of the following has maximum number of atoms of oxygen?
Thus there are two moles of atoms present in \[12.044 \times {10^{23}}\] molecules of carbon dioxide. Thus, the maximum number of oxygen atoms are present in \[12.044 \times {10^{23}}\] molecules of carbon dioxide and option (d) is correct.
What prevents an atom from being collapsed?
Hence, the movement of electrons in discrete energy levels prevents an atom from being collapsed.
How many atoms are in a human?
It is hard to grasp just how small the atoms that make up your body are until you take a look at the sheer number of them. An adult is made up of around 7,000,000,000,000,000,000,000,000,000 (7 octillion) atoms.
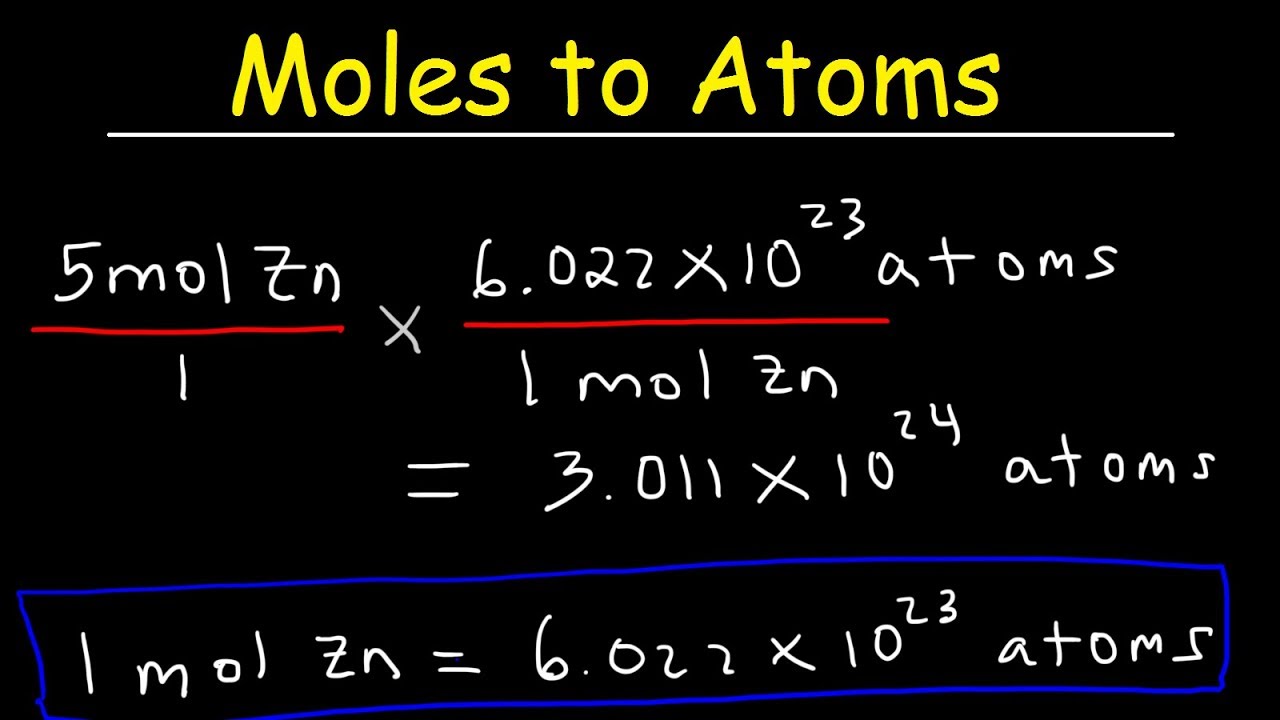
Moles To Atoms Conversion – Chemistry
Images related to the topicMoles To Atoms Conversion – Chemistry
How many atoms are there in a full stop?
Individual atoms are very small and have very little mass. There are about ten million million atoms in this full stop. An atom is made up of a nucleus that is surrounded by electrons.
Why are atoms in periods?
The horizontal rows are called periods. Periods correspond to the relationship of orbitals, or likely areas in which electrons will be found, inside the outermost shell of the atom. Successive periods down the table correspond to atoms with a more electron-rich core of inner shells.
Related searches
- what is the mass in grams of each of the following?
- what is the mass in grams of 3 011 x1023 atoms f
- calculate the number of atoms in each of the following
- determine the number of atoms in each of the following
- how many atoms are there in each of the following substances 5 28 g p
- how many atoms are there in each of the following substances 5.24 g p
- how many atoms are there in each of the following substances 5.28 g p
- determine the number of atoms in each of the following:
- how many atoms are there in each of the following 7.02 g si
- how many moles of atoms are there in each of the following
- how many moles of atoms are there in each of the following 40.1 g ca
- how many atoms are there in each of the following 7 02 g si
- how many atoms are there in each of the following substances 5.20 g p
- how many atoms of iron are there in 2.46 moles of each of the following
- how many moles of atoms of each element are there in one mole of the following compounds
- how many atoms are there in each of the following substances 5.12 g p
- what is the mass in grams of each of the following
- how many atoms are there in each of the following substances
- how many atoms are there in each of the following substances? 5.28 g p
- what is the mass in grams of 3.011 x10^23 atoms f
Information related to the topic how many atoms are there in each of the following
Here are the search results of the thread how many atoms are there in each of the following from Bing. You can read more if you want.
You have just come across an article on the topic how many atoms are there in each of the following. If you found this article useful, please share it. Thank you very much.
