Which has more dipole moment, CHCl3 or CH2Cl2?
The dipole moment of a molecule is a measure of its overall polarity. A molecule is considered polar if it has a separation of charge, meaning one end of the molecule is slightly positive and the other end is slightly negative. This separation of charge arises from the difference in electronegativity between the atoms in the molecule.
CH2Cl2 has a higher dipole moment than CHCl3 because the C-Cl bonds in CH2Cl2 are oriented in a way that their individual dipole moments reinforce each other. Think of it like this: imagine each C-Cl bond as a little arrow pointing towards the more electronegative chlorine atom. In CH2Cl2, these arrows are pointing in roughly the same direction, adding up to a larger overall dipole moment.
In contrast, the C-Cl bonds in CHCl3 are arranged in a tetrahedral shape. Because of the shape, the dipole moments of the C-Cl bonds partially cancel each other out, resulting in a smaller overall dipole moment for CHCl3.
To put it simply, the dipole moments in CH2Cl2 are working together to create a bigger effect, while the dipole moments in CHCl3 are partially canceling each other out. This is why CH2Cl2 has a higher dipole moment than CHCl3.
Let me know if you’d like to explore this further, I’m happy to break down the concepts in more detail.
Why is CH3Cl more polar than CH2Cl2?
The key lies in the dipole moments of these molecules. A dipole moment arises from the uneven distribution of electron density within a molecule, leading to a partial positive and partial negative end.
CH3Cl has a larger dipole moment than CH2Cl2 because the three hydrogen atoms and the chlorine atom in CH3Cl are arranged in a tetrahedral shape. This arrangement results in the polarity vectors of the hydrogen and chlorine atoms adding up, creating a significant dipole moment.
In CH2Cl2, the two chlorine atoms are positioned in a way that causes their polarity vectors to partially cancel each other out. This leads to a smaller dipole moment compared to CH3Cl.
Think of it like this: imagine pulling on a rope with two people. If both people pull in the same direction, the rope will be pulled with more force. But if they pull in opposite directions, the rope will be pulled with less force. The same principle applies to the dipole moments of CH3Cl and CH2Cl2.
The polarity vectors of the hydrogen and chlorine atoms in CH3Cl all point in roughly the same direction, creating a strong pull. However, in CH2Cl2, the chlorine atoms are pulling in somewhat opposing directions, resulting in a weaker overall pull.
This is why CH3Cl is more polar than CH2Cl2.
Why is dichloromethane more polar than chloroform?
The C-Cl and C-H bond lengths and angles play a crucial role in determining the overall polarity of a molecule. While the C-Cl bond is polar due to the difference in electronegativity between carbon and chlorine, the C-H bond is considered only slightly polar. In dichloromethane (CH2Cl2), the two C-Cl bonds are positioned in a way that their dipole moments reinforce each other, resulting in a greater overall dipole moment.
However, in chloroform (CHCl3), the three C-Cl bonds are positioned such that their dipole moments partially cancel each other out. This leads to a smaller overall dipole moment in chloroform compared to dichloromethane.
Think of it like this: imagine you have two people pulling on a rope in the same direction. That’s like the two C-Cl bonds in dichloromethane working together to create a stronger pull. Now, imagine three people pulling on a rope, but they’re not all pulling in the same direction. That’s similar to the three C-Cl bonds in chloroform, where their individual pulls are less effective due to the opposing forces.
So, even though chloroform has more C-Cl bonds, the net effect on its overall dipole moment is less than that of dichloromethane.
Why CHCl3 has less dipole moment?
The key lies in the bond angles and the geometry of the molecule. CHCl3, or chloroform, has a tetrahedral shape. You might think that with one hydrogen and three chlorine atoms, the molecule would have a significant dipole moment pointing towards the chlorine side. However, this isn’t the case.
Here’s why:
The bond angle between the chlorine atoms in CHCl3 (Cl-C-Cl) is larger than expected due to the size of the chlorine atoms. These large atoms push each other further apart, resulting in a greater bond angle. This pushes the hydrogen atom closer to the chlorine atoms, causing the dipole moments of the C-Cl bonds to partially cancel each other out.
Think of it like this: Imagine you have three people pushing on a door from the same side. If they stand close together, their force will be stronger. But, if they spread out, their individual forces might not be as powerful combined.
In contrast, the bond angle in CH4, or methane, is smaller than expected due to the smaller size of the hydrogen atoms. This results in a larger net dipole moment because the C-H bonds are oriented in a way that their dipole moments add up.
Let’s break it down further:
Dipole Moment: A dipole moment is a measure of the separation of positive and negative charges in a molecule. A larger dipole moment indicates a greater separation of charge.
Bond Angle: The angle between two bonds that share a common atom.
Geometry: The overall shape of a molecule.
Therefore, the larger bond angle in CHCl3 leads to a decrease in the net dipole moment, making it smaller than what you might initially expect.
Why does CH3Cl have the highest dipole moment?
It all boils down to the fact that chlorine has a higher electron affinity than fluorine. This means chlorine is more eager to grab electrons and hold onto them. Because of this, the charge separation between the carbon and chlorine in CH3Cl is greater than the charge separation between the carbon and fluorine in CH3F.
Think of it like this: chlorine is a bit of a hog when it comes to electrons. It pulls more strongly on the shared electrons in the C-Cl bond, making the carbon slightly positive and the chlorine slightly negative. This creates a bigger dipole moment, a measure of how much separation there is between positive and negative charges.
You might wonder why this happens, since fluorine is the most electronegative element. While fluorine is indeed the most electronegative, meaning it has a strong pull on electrons, the electron affinity is different. Electron affinity is a measure of how much energy is released when an atom gains an electron.
While fluorine is great at pulling electrons, it already has a strong hold on its own electrons, so it doesn’t gain additional ones as readily. Chlorine, on the other hand, is more willing to gain an electron because its outermost shell isn’t as tightly packed.
This leads to the C-Cl bond having a greater polarity, which in turn contributes to the higher dipole moment of CH3Cl.
Does CH2Cl2 have zero dipole moment?
Let’s break down why this happens. Dichloromethane has a central carbon atom, bonded to two hydrogen atoms and two chlorine atoms. The chlorine atoms are more electronegative than the carbon and hydrogen atoms, meaning they pull the shared electrons in the bonds closer to themselves. This creates a partial negative charge on the chlorine atoms and a partial positive charge on the carbon and hydrogen atoms.
The geometry of the molecule plays a crucial role in determining the dipole moment. Dichloromethane has a tetrahedral shape, with the chlorine atoms located at two opposite corners of the tetrahedron. This arrangement means that the chlorine atoms are on the same side of the molecule, and the hydrogen atoms are on the opposite side. Since the chlorine atoms are more electronegative, the molecule has a net dipole moment pointing towards the chlorine atoms.
Let’s visualize this. Imagine the chlorine atoms as two small, negatively charged magnets, and the hydrogen atoms as two small, positively charged magnets. These magnets are arranged in a way that the chlorine magnets are on the same side of the molecule, creating a stronger pull in that direction.
The net dipole moment is a measure of this overall separation of charge. In dichloromethane, the dipole moment is 1.6 Debye, indicating a significant separation of charge, which is why dichloromethane is a polar molecule.
Polar molecules are important in chemistry because they can interact with other polar molecules through dipole-dipole interactions. These interactions are responsible for many properties of polar molecules, such as their higher boiling points and solubility in polar solvents.
See more here: What Is The Dipole Moment Of Chloroform? | Dipole Moment Of Chloroform And Dichloromethane
What is the dipole moment of chloromethane?
Chloromethane has a dipole moment of 1.87 D (Debye), while dichloromethane has a dipole moment of 1.60 D. So, while dichloromethane has two polar C-Cl bonds, chloromethane only has one. The reason for this seemingly contradictory observation lies in the geometry of the molecules.
Chloromethane has a tetrahedral shape, with the chlorine atom positioned at one vertex. This arrangement creates a net dipole moment due to the difference in electronegativity between carbon and chlorine. The chlorine atom, being more electronegative, pulls electron density towards itself, creating a partial negative charge on the chlorine side and a partial positive charge on the carbon side.
Dichloromethane, on the other hand, has a bent geometry due to the presence of two chlorine atoms. While both C-Cl bonds are polar, their dipole moments partially cancel each other out due to their opposing directions.
Therefore, even though dichloromethane has two polar bonds, the vector sum of the individual bond dipoles is smaller than the dipole moment of chloromethane.
Now, let’s look at the boiling points. Dichloromethane has a higher boiling point than chloromethane despite having a lower dipole moment. This can be explained by the van der Waals forces that arise from temporary fluctuations in electron distribution.
Dichloromethane, with its larger molecular size and two chlorine atoms, experiences stronger van der Waals forces compared to chloromethane. These forces are responsible for holding the molecules together in the liquid state and require more energy to overcome, hence the higher boiling point.
In summary, the dipole moment is influenced by the geometry and polarity of the molecule, while boiling point is influenced by the strength of intermolecular forces. These forces, primarily van der Waals forces in this case, play a crucial role in determining the physical properties of molecules.
Why is dichloromethane more polar than chloroform?
First, dichloromethane (CH₂Cl₂) has two chlorine atoms attached to the central carbon atom, while chloroform (CHCl₃) has three. These chlorine atoms are more electronegative than carbon and hydrogen, meaning they pull the shared electrons in the bonds towards themselves. This creates a partial negative charge on the chlorine atoms and a partial positive charge on the carbon atom.
But here’s the key: the chlorine atoms in dichloromethane are arranged symmetrically, meaning they are on opposite sides of the carbon atom. This creates a larger dipole moment, which is a measure of the molecule’s polarity. In chloroform, the three chlorine atoms are arranged in a triangular shape, so their individual dipole moments partially cancel each other out, resulting in a smaller overall dipole moment.
Essentially, in dichloromethane, the two chlorine atoms are pulling electrons in opposite directions, creating a strong overall dipole. In chloroform, the three chlorine atoms are pulling electrons in different directions, partially canceling each other out, leading to a weaker overall dipole.
In addition to the number of chlorine atoms, the bond angles also play a role. Dichloromethane has a tetrahedral shape, meaning the bond angles between the chlorine atoms are 109.5 degrees. This maximizes the separation of the chlorine atoms, further enhancing the dipole moment. In contrast, chloroform has a slightly distorted tetrahedral shape, with bond angles of around 107 degrees. This slight distortion reduces the separation of the chlorine atoms and contributes to a weaker dipole moment.
Therefore, the combination of a larger number of chlorine atoms, a more symmetrical arrangement, and a more favorable bond angle in dichloromethane leads to a stronger dipole moment compared to chloroform, making dichloromethane more polar.
What is a molecular dipole moment?
Let’s say you have a molecule with more than one polar bond. These are bonds where electrons are shared unequally between atoms, creating a slight separation of charge within the bond. Each bond dipole moment is like a tiny arrow pointing from the slightly negative end of the bond to the slightly positive end.
The molecular dipole moment is the sum of all these tiny bond dipole moment arrows. Think of it like adding vectors. You need to consider both the strength (magnitude) of each arrow and the direction it’s pointing.
If the arrows mostly cancel each other out, the molecule is considered nonpolar. Think of it like a tug-of-war where both teams are equally strong – there’s no net movement.
But if the arrows don’t cancel each other out, the molecule is polar. Think of it like a tug-of-war where one team is stronger – there’s a net movement in one direction.
Let me give you a few examples to illustrate this:
Carbon dioxide (CO2) has two polarC=O bonds. However, the two bonds are perfectly symmetrical and point in opposite directions. Therefore, the molecular dipole moment is zero, making CO2 a nonpolar molecule.
Water (H2O) has two polarO-H bonds. These bonds are not symmetrical and don’t point in opposite directions. As a result, the molecular dipole moment is not zero, making H2O a polar molecule.
It’s important to understand the concept of molecular dipole moment because it directly impacts a molecule’s properties, like its melting point, boiling point, and solubility. Understanding the forces involved helps us understand the behavior of molecules and materials.
Does CHCl 3 have a dipole moment?
CHCl3 has a tetrahedral geometry, meaning the carbon atom is at the center, and the hydrogen and chlorine atoms are positioned at the corners of a tetrahedron. Now, even though the molecule has this symmetrical shape, the atoms attached to the carbon atom are not identical. We have one hydrogen atom and three chlorine atoms.
This difference in atoms leads to an interesting phenomenon. The bond dipole moments, which represent the direction and magnitude of the electrical polarity of a bond, don’t cancel each other out. Instead, they add up, resulting in a net dipole moment for the entire CHCl3 molecule.
Think of it like this: chlorine is more electronegative than hydrogen, meaning it attracts electrons more strongly. This creates a slight negative charge on the chlorine atoms and a slight positive charge on the hydrogen atom. Because there are three chlorine atoms and only one hydrogen atom, the overall effect is a dipole moment pointing towards the chlorine atoms.
Let’s break down why CHCl3 exhibits a dipole moment:
1. Tetrahedral Geometry: The structure of CHCl3 is a tetrahedron, with the carbon atom at the center and the other atoms at the corners. This geometry is important for understanding how the bond dipole moments interact.
2. Non-Identical Atoms: The presence of three chlorine atoms and one hydrogen atom creates an imbalance in the electronegativity of the molecule. Chlorine, being more electronegative, attracts electrons more strongly than hydrogen.
3. Non-Cancellation of Bond Dipole Moments: Due to the difference in electronegativity and the arrangement of atoms, the bond dipole moments don’t cancel each other out. Instead, they add up, resulting in a net dipole moment for the molecule.
4. Net Dipole Moment: The direction of the dipole moment in CHCl3 is towards the chlorine atoms, as they have a stronger pull on the electrons.
In essence, the presence of three chlorine atoms, which are more electronegative than hydrogen, and the tetrahedral geometry of the molecule create a net dipole moment in CHCl3. This means CHCl3 is considered a polar molecule, which has significant implications for its chemical properties and interactions with other molecules.
See more new information: countrymusicstop.com
Dipole Moment Of Chloroform And Dichloromethane: A Comparison
Understanding Dipole Moments
Imagine a molecule like a tug-of-war, with different parts pulling on the electrons. Electronegativity, the ability of an atom to attract electrons, is like the strength of each player in the tug-of-war.
If one side has a stronger pull, we get an uneven distribution of electrons, creating a dipole moment – a separation of charge within the molecule. Think of it like a tiny magnet with a positive and negative end.
Chloroform (CHCl3)
Now, let’s look at chloroform (CHCl3). Here’s the deal: Chlorine (Cl) is more electronegative than hydrogen (H) or carbon (C). So, the chlorine atoms pull electrons toward themselves. This creates a partial negative charge (δ-) on the chlorine side and a partial positive charge (δ+) on the hydrogen side.
The molecule has a tetrahedral shape, but since the chlorine atoms are all on one side, the dipole moments don’t cancel each other out. This results in a net dipole moment for chloroform.
Dichloromethane (CH2Cl2)
Now, let’s move on to dichloromethane (CH2Cl2). It’s similar to chloroform in that chlorine is more electronegative than hydrogen or carbon. But, it has two chlorine atoms, pulling electrons in opposite directions.
Since the geometry is tetrahedral, the two C-Cl bonds, with their individual dipole moments, partially cancel each other out. This means dichloromethane still has a dipole moment, but it’s smaller than that of chloroform.
The Bottom Line
The takeaway here is that chloroform has a larger dipole moment than dichloromethane due to the greater number of chlorine atoms pulling electrons in the same direction.
This difference in dipole moment can affect things like solubility and boiling point. For instance, chloroform is more soluble in polar solvents like water, while dichloromethane is more soluble in nonpolar solvents.
FAQs
Here are some common questions about dipole moments that people often ask.
1. What is the dipole moment of chloroform?
The dipole moment of chloroform is 1.01 D.
2. What is the dipole moment of dichloromethane?
The dipole moment of dichloromethane is 1.6 D.
3. Why is the dipole moment of chloroform smaller than dichloromethane?
The dipole moment of dichloromethane is larger than chloroform because the chlorine atoms are pulling electrons in opposite directions in dichloromethane. This partially cancels out the dipole moments.
4. How does dipole moment affect the boiling point of a molecule?
Molecules with larger dipole moments tend to have higher boiling points. This is because the stronger dipole-dipole interactions between molecules require more energy to overcome.
5. How does dipole moment affect the solubility of a molecule?
Molecules with larger dipole moments are more soluble in polar solvents, such as water. This is because they can form stronger hydrogen bonds with the solvent molecules.
Summary
Understanding dipole moments can help us make sense of how molecules behave. By comparing chloroform and dichloromethane, we see how the number and arrangement of polar bonds can influence a molecule’s dipole moment and its physical properties.
Dipole moment of CH2Cl2 and CHCl3 – Chemistry Stack Exchange
As the dipolar moment is a vectorial property, you have to take into account both the number of chlorine atoms around the carbon atom, as well as their relative arrangement — among them, as well in respect to the carbon atom. Chemistry Stack Exchange
Why does chloromethane have a larger dipole
Why does $\ce{CH3Cl}$, methyl chloride, have a larger dipole moment than $\ce{CHCl3}$, chloroform? Let us consider Chemistry Stack Exchange
7.5: Dipole Interactions – Chemistry LibreTexts
The dipole moment of chloromethane, 12.9 D, is higher than that of dichloromethane, 9.08 D, even though dichloromethane has a Chemistry LibreTexts
Difference of dipole moments of dichloromethane and
Consider each $\ce{C-Cl}$ bond, which has a bond dipole moment of magnitude $A$. The contribution from $\ce{C-H}$ is neglected here to simplify the calculations. Now consider Chemistry Stack Exchange
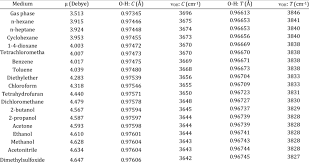
Solvent Physical Properties – UMass
33 rows Values Taken from UNIVERSITY OF MASSACHUSETTS AMHERST
9.11: Polar Covalent Bonds – Dipole Moments – Chemistry
Individual bond dipole moments are indicated in black. Due to their different three-dimensional structures, some molecules with polar bonds have a net dipole Chemistry LibreTexts
Dipole moment—Organic compounds – ScienceDirect
This chapter presents the results of the dipole moment for organic compounds in tabular format. The tabulation is arranged by carbon number such as C, ScienceDirect
1.9.3: Dipole moments – Chemistry LibreTexts
Dipole moments tell us about the charge separation in a molecule. The larger the difference in electronegativities of bonded atoms, the larger the dipole moment. For example, NaCl Chemistry LibreTexts
Phase Stability of Chloroform and Dichloromethane
Chloroform (CHCl3) and dichloromethane (CH2Cl2) are model systems for the study of intermolecular interactions, such as hydrogen bonds and halogen–halogen interactions. Here we report a MDPI
Dipole Moment – LSU
Dipole Moment. Burdick & Jackson solvents are arranged in order of increasing dipole moment, the mathematical product of the distance between the centers of charge in the Macromolecular Studies Group
Is Chcl3 Polar Or Nonpolar? (Trichloromethane Or Chloroform)
Chloroform And Di Chloromethane Dipole Moment
Is Ch2Cl2 Polar Or Nonpolar? (Dichloromethane)
Dipole Moment, Vectors, \U0026 Electronegativity – Organic Chemistry
Special Problem – Dipole Moment Comparison
Link to this article: dipole moment of chloroform and dichloromethane.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
