Is isobutyl alcohol and sec-butyl alcohol the same?
So, are isobutyl alcohol and sec-butyl alcohol the same? The answer is no, they are not the same. They have different arrangements of their carbon atoms, which is what makes them distinct.
Think of it like this: Imagine you have four building blocks, each representing a carbon atom. You can arrange these blocks in different ways to create unique structures. In the case of isobutyl alcohol, one carbon atom has three other carbon atoms attached to it, like a tree trunk with three branches. This is called a branched structure. In contrast, sec-butyl alcohol has a linear chain structure, where the carbon atoms are connected in a row, with a slight “bend” in the middle.
These structural differences might seem subtle, but they lead to variations in how these alcohols interact with other molecules. The way the carbon atoms are arranged influences the reactivity of the molecule and the types of products that can be formed.
To illustrate this: Let’s consider a simple reaction: oxidation. When you oxidize isobutyl alcohol, you’ll get a different product than when you oxidize sec-butyl alcohol. The specific products depend on the reaction conditions, but the point is that the different structures influence the outcome.
This difference in reactivity is a key concept in organic chemistry. It’s important to recognize that, even with the same molecular formula, different structures can lead to vastly different properties and applications.
What is the difference between isobutyl and tert butyl structure?
We can create different alkyl groups by removing a hydrogen atom from different carbon atoms in a parent alkane. When we remove a hydrogen atom from a primary carbon atom of isobutane, we get the isobutyl group. If we remove a hydrogen atom from the tertiary carbon atom of isobutane, we get the tert-butyl group.
The key here is understanding the difference between primary, secondary, and tertiary carbon atoms. A primary carbon atom is directly bonded to only one other carbon atom. A secondary carbon atom is directly bonded to two other carbon atoms. A tertiary carbon atom is directly bonded to three other carbon atoms.
In isobutane, the central carbon is a tertiary carbon, and it is bonded to three other carbon atoms. So, when we remove a hydrogen atom from this central carbon, we get a tert-butyl group.
The isobutyl group, on the other hand, is derived from a primary carbon atom. It’s important to note that the isobutyl group is a primary alkyl group, even though it is derived from a branched alkane. This is because the carbon atom that is attached to the main chain is a primary carbon atom.
In summary, the difference between isobutyl and tert-butyl groups is the location of the carbon atom from which the hydrogen atom is removed. Isobutyl is derived from a primary carbon atom, while tert-butyl is derived from a tertiary carbon atom. This difference results in different branching patterns and connectivity in the alkyl groups.
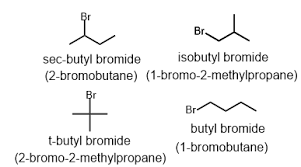
What is the difference between isobutyl bromide and secondary butyl bromide?
Isobutyl bromide gets its name because its structure is branched at the second carbon of the chain. This means that one of the carbon atoms in the chain is attached to three other carbon atoms. Imagine a “Y” shape, where the point of the Y is the second carbon atom in the chain.
Secondary butyl bromide, on the other hand, has its branch at the first carbon atom in the chain. This means that the first carbon is connected to two other carbon atoms, creating a sort of “L” shape.
Here’s a way to visualize it:
Isobutyl Bromide: Think of a Y shape. The point of the Y is the second carbon of the chain, and the bromine is attached to the end of one of the arms.
Secondary Butyl Bromide: Think of an L shape. The corner of the “L” is the first carbon of the chain, and the bromine is attached to the end of the long side of the “L”.
Remember, the bromine atom is always attached to a carbon atom in these compounds. The difference lies in how those carbon atoms are connected to each other – that “branching” is what gives each isomer its unique properties and name.
What is the difference between ISO and SEC alcohol?
Iso is a prefix used to describe a specific type of alcohol molecule. You’ll see this prefix when the alcohol’s structure has a carbon chain with six or fewer carbon atoms. The key is that one of these carbon atoms is attached to the second-to-last carbon in the chain. Think of it like a little branch sticking out from the main chain.
Sec (short for secondary) is used to describe a slightly different kind of alcohol. This prefix tells us that the functional group, which is the -OH (hydroxyl) group, is attached to a secondary carbon atom. What does that mean? A secondary carbon atom is one that is bonded to two other carbon atoms.
It’s important to note that the sec prefix is typically only used when the carbon chain has at least four carbon atoms.
Example
Let’s use an example to make things clear:
2-methylpropan-2-ol is an iso alcohol. It has four carbons in the chain (propane), and a methyl group (CH3) is attached to the second carbon from the end.
2-butanol is a sec alcohol. It has four carbon atoms in the chain (butane), and the hydroxyl group is attached to the second carbon atom in the chain.
To recap, iso and sec are prefixes used to specify the structure of alcohols. The iso prefix indicates a branched structure, while the sec prefix indicates that the hydroxyl group is attached to a secondary carbon atom.
What’s the difference between isobutyl and sec-butyl?
Both isobutyl and sec-butyl are four-carbon groups. However, the way those carbons are connected makes a difference. Isobutyl has a branched structure at the second carbon, while sec-butyl has a branched structure at the first carbon. Let me explain:
Isobutyl looks like this:
“`
CH3
|
CH3-CH-CH2
|
H
“`
Notice how the second carbon (from left to right) is attached to three other carbons. This makes it a “branch point”.
Sec-butyl looks like this:
“`
CH3
|
CH3-CH2-CH
|
H
“`
In sec-butyl, the first carbon is attached to three other carbons, making it the branch point.
Key Takeaway: The difference between isobutyl and sec-butyl is where that single “branch” occurs in the four-carbon chain.
Let me know if that helps!
What is the difference between ISO and SEC?
Iso is used to name an organic compound where all carbon atoms, except one, form a continuous chain. Think of it like a straight line with a small branch sticking out. For example, isobutane has a main chain of three carbon atoms with a single carbon atom branching off the second carbon.
Sec, on the other hand, identifies a functional group attached to a secondary carbon atom. A secondary carbon atom is bonded to two other carbon atoms.
Here’s a simple analogy: Imagine a tree. The trunk represents the main chain of carbon atoms. The branches represent the side chains or functional groups. If a branch grows directly from the trunk (primary carbon), we don’t use iso or sec. But if a branch grows from another branch (secondary carbon), we use sec.
Let’s dive a little deeper into the concept of secondary carbon atoms.
A secondary carbon atom is a carbon atom that is directly bonded to two other carbon atoms. This distinction is crucial because it influences the reactivity of the molecule. For example, if a sec-butyl group is attached to an alcohol, it will have different properties than if a tert-butyl group (a tertiary carbon) were attached. The sec designation highlights the specific location of the functional group, allowing us to predict and understand the molecule’s behavior.
In conclusion, iso and sec are prefixes used to describe the structure of organic molecules, specifically how carbon chains are arranged and where functional groups are attached. Understanding these prefixes is essential for accurately naming and predicting the properties of organic compounds.
What is a suitable test to distinguish between isobutyl alcohol and t-butyl alcohol?
Here’s how it works:
Tertiary alcohols react immediately with the Lucas reagent at room temperature, forming a cloudy white precipitate of the corresponding alkyl halide. This is because the tertiary carbocation formed is very stable.
Secondary alcohols react with the Lucas reagent at a slower rate, typically taking 3-5 minutes to show turbidity. This is because the secondary carbocation formed is less stable than a tertiary carbocation.
Primary alcohols react very slowly with the Lucas reagent, and the reaction may not occur at all at room temperature. This is because the primary carbocation formed is the least stable of the three.
t-Butyl alcohol is a tertiary alcohol, while isobutyl alcohol is a primary alcohol. Therefore, t-butyl alcohol will react immediately with the Lucas reagent at room temperature, forming a cloudy white precipitate. Isobutyl alcohol will not react at all, or will react very slowly, at room temperature.
The Lucas test is a simple and effective way to distinguish between primary, secondary, and tertiary alcohols. It is based on the difference in reactivity of these alcohols with the Lucas reagent. This is because the rate of reaction depends on the stability of the carbocation intermediate formed during the reaction.
See more here: What Is The Difference Between Isobutyl And Tert Butyl Structure? | Difference Between Isobutyl And Sec Butyl
What is the difference between isobutyl & sec-butyl?
They might sound similar, but their branching structures give them distinct characteristics. Isobutyl has a branched structure, like a little tree, with a methyl group (CH3) attached to the second carbon atom. Sec-butyl, on the other hand, has a straight chain structure with a methyl group attached to the second carbon atom.
Think of it like this: isobutyl is like a fork, with one prong branching out, while sec-butyl is like a straight line.
This seemingly small difference in structure can lead to different chemical and physical properties for molecules containing these groups. For example, isobutyl alcohol is a common solvent, while sec-butyl alcohol is used in the production of certain plastics.
Here’s a more visual breakdown:
Isobutyl
“`
CH3
|
CH3-CH-CH2-
|
H
“`
Sec-butyl
“`
CH3-CH2-CH-CH3
|
H
“`
As you can see, the methyl group’s position in the structure is the key factor differentiating the two. This seemingly minor structural change can significantly affect the reactivity and properties of molecules containing these groups.
Why do isobutyl & sec-butyl have different chemical reactivity?
For example, in a nucleophilic substitution reaction, a nucleophile can easily swap places with the methyl group on isobutyl. This is because the methyl group isn’t tightly bound and is relatively easy to kick off.
Sec-butyl, on the other hand, has a slightly different structure. It lacks that readily available methyl group. Instead, it has a secondary carbon attached to the butyl group. This makes it less likely to participate in substitution reactions. The secondary carbon is more stable and less prone to being replaced by a nucleophile.
Let’s visualize this with a simple analogy: Imagine you have two toys, a simple building block and a complex car. The building block (like the methyl group) is easy to swap out for a different block. The car (like the secondary carbon), however, is more complex and harder to take apart and replace. The secondary carbon is similar to the car; it’s more stable and less likely to be replaced in a reaction.
So, in a nutshell, isobutyl with its methyl group is like a simple building block, ready to be swapped out in substitution reactions. Sec-butyl with its secondary carbon, on the other hand, is like a complex car, less prone to replacement. This difference in structure is why isobutyl and sec-butyl exhibit different chemical reactivities.
What is sec-butyl cyclopentane?
Let’s break down sec-butyl cyclopentane step by step. You might see it written as s-butylcyclopentane too, it’s the same thing. It’s a cycloalkane with a sec-butyl group attached.
Think of it like this: cyclopentane is a ring of five carbon atoms. Imagine a five-sided shape. Now, sec-butyl is a branch, a group of four carbon atoms. One of those four carbon atoms is attached to the ring. That’s what makes this compound sec-butyl cyclopentane.
The “sec” in sec-butyl stands for secondary. That means the carbon atom that’s connected to the ring is attached to two other carbon atoms in the butyl group. It’s a bit like a crossroads in a chain of carbon atoms.
sec-butyl is a specific type of butyl group. Butyl always means there are four carbon atoms. The ‘sec’ tells us how it’s attached. There are actually a few different ways a butyl group can attach, each with its own name:
n-butyl: This means the four carbon atoms are in a straight chain, like a single-lane road.
iso-butyl: This one is a branched chain with a ‘T’ shape.
tert-butyl: This one is like a ‘Y’ shape, with the carbon atom connected to the ring sitting at the junction.
sec-butyl cyclopentane is just one of many possible combinations of cycloalkanes and different types of alkyl groups. These combinations create a huge variety of organic compounds with their own unique properties and uses.
How does a sec-butyl group differ from a tertiary carbon?
Sec-butyl, a four-carbon group, has its branching at the second carbon atom, which gives it a unique molecular shape. On the other hand, isobutyl has its branching point leading to a tertiary carbon connected to three other carbons.
Think of it like this: sec-butyl’s branching point forms a secondary carbon attached to two other carbons and one hydrogen atom.
To understand this better, visualize the structure of a sec-butyl group. The main chain has four carbons, and the branching occurs on the second carbon, making it a secondary carbon. This means the second carbon is connected to two other carbons and a hydrogen atom.
Now, let’s look at a tertiary carbon. In a tertiary carbon, the carbon atom is directly attached to three other carbon atoms. This means it has no hydrogen atoms directly bonded to it.
The difference between sec-butyl and a tertiary carbon lies in the branching point and the number of carbons attached to the branching carbon. Sec-butyl has a secondary carbon at its branching point, while a tertiary carbon has a carbon attached to three other carbons. This difference in structure significantly impacts the properties and reactivity of molecules containing these groups.
See more new information: countrymusicstop.com
Difference Between Isobutyl And Sec-Butyl: A Clear Explanation
So, you’re trying to figure out the difference between isobutyl and sec-butyl, huh? It’s a common question, especially if you’re working with organic chemistry or dealing with different types of molecules.
Let’s break it down.
The Basics: What are Isobutyl and Sec-Butyl?
These terms refer to specific isomers, which are molecules with the same chemical formula but different arrangements of atoms.
In this case, both isobutyl and sec-butyl have the same formula: C4H9. That means they both have four carbon atoms and nine hydrogen atoms.
But, the way those atoms are connected makes all the difference.
Visualizing the Difference
Here’s a simple way to picture it:
Isobutyl: Imagine a chain of four carbon atoms. The first carbon atom has three hydrogen atoms attached to it. The second and third carbon atoms each have two hydrogen atoms. And the final carbon atom has one hydrogen atom. This structure forms a branched chain where the last carbon atom branches off from the main chain.
Sec-butyl: Again, imagine a chain of four carbon atoms. But this time, the second carbon atom has three hydrogen atoms attached to it. The first and third carbon atoms each have two hydrogen atoms. The final carbon atom has one hydrogen atom. This creates a linear chain with a substituent on the second carbon.
Key Differences
Structure: Isobutyl is branched, while sec-butyl is a linear chain with a substituent.
Functional Group: Both are alkyl groups, meaning they can attach to a larger molecule. However, the way they attach and how they affect the larger molecule can be different due to their different structures.
Properties: The different structures of isobutyl and sec-butyl can lead to subtle variations in properties like boiling point, reactivity, and solubility.
Why Understanding This Matters
So, why is it important to know the difference? Well, it comes down to functional groups and the ways they interact.
For example, if you’re working with isobutyl alcohol, it will have different properties compared to sec-butyl alcohol.
The way these groups connect and interact can influence things like:
Chemical reactions: How readily a molecule reacts with other substances.
Physical properties: Boiling point, melting point, and solubility.
Biological activity: How a molecule interacts with living systems.
Example: Isobutyl vs. Sec-Butyl Alcohol
Isobutyl alcohol: A colorless liquid used in making perfumes, flavors, and pharmaceuticals.
Sec-butyl alcohol: Also a colorless liquid, used in paints, resins, and as a solvent.
Understanding the Difference:
While both alcohols have the same formula (C4H10O), they have different structures and thus, different properties. The difference is subtle, but it can make a big difference in how these alcohols are used.
Key Takeaways
Isobutyl and sec-butyl are isomers with the same chemical formula but different structures.
Isobutyl is branched, while sec-butyl is a linear chain with a substituent.
* These differences in structure can affect the properties and reactivity of molecules.
FAQs
Q: How do I know if a molecule is isobutyl or sec-butyl?
A: Look at the structure of the molecule. Isobutyl will have a branched chain with the branching point on the second carbon atom. Sec-butyl will have a linear chain with a substituent on the second carbon atom.
Q: Why are these two groups so similar?
A: They’re similar because they have the same chemical formula, meaning they have the same number of carbon and hydrogen atoms. The difference lies in the arrangement of those atoms.
Q: Are there other types of butyl groups?
A: Yes! There are three other types: n-butyl, tert-butyl, and neopentyl. Each has a different structure and therefore different properties.
Q: How do I learn more about isobutyl and sec-butyl?
A: A good textbook on organic chemistry can help! You can also find plenty of resources online that explain the differences between these groups and their related compounds.
Isobutyl vs. Sec-butyl – What’s the Difference? | This vs. That
Learn how isobutyl and sec-butyl differ in their chemical structure, physical properties, and chemical reactivity. See examples of their applications in various industries and compare their common and systematic names. thisvsthat.io
Isobutyl vs. Sec-butyl: What’s the Difference?
Learn how isobutyl and sec-butyl differ in structure, reactivity, and applications. See comparison chart, definitions, FAQs, and examples of these four Difference Wiki
What is the difference between isobutyl and sec
The structure of sec-butyl is shown below: Isobutyl forms isobutyl compounds such as isobutyl alcohol. Sec-butyl forms secondary-carbon compounds such as secondary alcohol. BYJU’S
Isobutyl vs. Sec-butyl — What’s the Difference?
Isobutyl and sec-butyl groups are both four-carbon branches derived from butane, but differ in their structure. Isobutyl has a primary carbon attached to a Ask Difference
Butyl group – Wikipedia
Butyl group. In organic chemistry, butyl is a four- carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers ( n -butane Wikipedia
Don’t Be Futyl, Learn The Butyls – Master Organic
The problem comes from the way we write these compounds: i-PrOH, s-BuOH and t-BuOH, which mean isopropyl alcohol, sec-butyl alcohol and tert-butyl alcohol, all correct names, where i Master Organic Chemistry
What is the difference between isobutyl and sec
Hence, the basic difference between isobutyl and sec-butyl is the arrangement of carbon atoms to the parent molecule. Note: Both compounds namely isobutyl and sec-butyl can react with the Vedantu
Naming Butyls – What does n-, s-, t- Mean?
Learn how to name the four forms of butyl group: n-, s-, t-, and isobutyl. See the common and systematic names for each form and their structures. ThoughtCo
Alkane Nomenclature 3 – Sec, Iso, Tert, Neo Naming
Isobutyl Secondary Butyl And Isopropyl Secondary Propyl Are Same Or Different ?
Common Names: Iso, Sec, Tert, Neo, N | Organic Chemistry
006 Alkyl Substituent Names And Structures
Naming Iso, Sec, \U0026 Tert R-Groups
Link to this article: difference between isobutyl and sec butyl.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
