Is a non-metal an insulator?
Think of it this way: Imagine a crowded dance floor. If everyone is tightly packed and holding onto each other, it’s difficult to move around, right? That’s similar to what happens in a non-metal. The electrons are like dancers, and the atoms are like the people holding onto each other. The electrons can’t move freely, so electricity can’t flow easily.
Now, let’s explore this a little further. While non-metals are generally good insulators, there are a few exceptions. For example, graphite, a form of carbon, is a good conductor of electricity. This is because graphite has a unique structure where its atoms are arranged in layers, allowing electrons to move freely between these layers.
Another interesting fact is that silicon and germanium, which are considered metalloids, can be made to behave like conductors under certain conditions. These materials are called semiconductors, and they play a crucial role in modern electronics.
So, while non-metals are generally good insulators, there are exceptions, and some elements can exhibit both insulating and conducting properties depending on their structure and conditions.
Are all non-metals insulators True or false?
Let’s dive a little deeper. Nonmetals are elements that lack the characteristic metallic luster, malleability, and ductility of metals. They tend to be found on the right side of the periodic table. A key feature of nonmetals is their tendency to gain electrons, making them good at forming negative ions.
Now, here’s the catch: while many nonmetals are excellent insulators, there are a few that behave differently. Graphite, for example, is a nonmetal that’s a surprisingly good conductor of electricity. This unique property is due to its layered structure, where electrons can easily move between the layers, allowing for electrical conductivity.
So, while it’s generally accurate to say that nonmetals are insulators, it’s crucial to remember that there are some exceptions like graphite. Understanding these nuances is key to appreciating the fascinating world of chemistry!
Are all non-metals conductors?
Let’s delve a bit deeper into why some nonmetals conduct electricity. The ability of a material to conduct electricity depends on the availability of free electrons. Metals have a “sea” of freely moving electrons, which allows them to carry an electrical current easily. Nonmetals, on the other hand, typically hold their electrons tightly within their atomic structure. This is why most nonmetals are insulators, like rubber or glass.
However, the structure of some nonmetals allows for the movement of electrons under certain circumstances. Graphite has a unique structure where its carbon atoms are arranged in layers. Within these layers, electrons are able to move freely, making graphite a good conductor. Silicon and metalloids also have interesting electronic structures that allow them to conduct electricity under certain conditions. These conditions might include specific temperatures or the presence of impurities. This is why semiconductors are so important in electronics, as their conductivity can be carefully controlled to build transistors, diodes, and other essential components.
Are all non conductors insulators?
Let’s break it down:
Conductors: These materials allow electricity to flow through them easily. Think of copper wires, which are great conductors, allowing electricity to travel through them without much resistance.
Insulators: These materials resist the flow of electricity. Think of the rubber coating on electrical wires. This coating prevents electricity from escaping and causing a shock. It’s like building a wall around the wire to keep the electricity inside.
Semiconductors: These materials are somewhere in between conductors and insulators. They can be made to conduct electricity under certain conditions, such as by applying a voltage or changing their temperature. This makes them really useful for building electronic devices like transistors and solar cells.
Think of it this way:
Conductors are like open highways, allowing electricity to flow freely.
Insulators are like closed roads, blocking the flow of electricity.
Semiconductors are like roads that can be opened or closed depending on the situation, allowing for controlled flow of electricity.
So, while insulators are a subset of non-conductors, they are not the only kind. Non-conductors can have different properties, and some, like semiconductors, have the ability to conduct electricity under specific conditions. This flexibility makes them valuable components in modern electronics.
Can an insulator be metal?
You’re right, we usually think of metals as good conductors of electricity. That’s because their electrons are free to roam around, allowing for easy current flow. Insulators on the other hand, hold their electrons tightly, preventing easy current flow.
Now, here’s the twist! Under specific conditions, metals can become insulators. This is where Hemley and Naumov’s discovery comes in. They found that when the spacing between atoms in a metal gets really close, and this close spacing happens in a specific way, the electrons become more localized. This means they are less free to roam around. Think of it as the electrons getting trapped between the atoms, no longer free to flow as they do in the usual metallic form.
Let’s break down this “specific way” a bit more:
Symmetry: It’s not just about the reduced spacing; it’s about the arrangement of atoms. The atoms need to be organized in a specific asymmetric way. This asymmetry disrupts the usual flow of electrons and traps them.
Localization: In normal metals, electrons are free to wander throughout the entire material. But with this specific asymmetric arrangement, electrons become localized, meaning they are confined to specific regions around the atoms. This localized nature prevents them from freely moving and conducting electricity, effectively turning the metal into an insulator.
Essentially, the conditions create a situation where the electrons are forced into a more stable, localized state. It’s like forcing electrons into a tiny box, preventing them from moving freely.
This discovery is really cool, showing that even materials we think are fixed in their properties (like metals always being conductive) can actually behave differently under specific conditions. It opens up exciting avenues for exploring and potentially designing new materials with unique properties.
Which metal is not an insulator?
You might be wondering why Mercury acts so differently. It all comes down to how its electrons are arranged. Metals are good conductors because their electrons are loosely bound to their atoms and can move freely, allowing electricity to flow easily. However, in Mercury, the electrons are held more tightly, making it difficult for them to move and carry an electrical current. This is why Mercury is considered a poor conductor and acts more like an insulator.
So, while Mercury is a metal, its unique electron structure makes it an exception to the rule. It’s a great example of how different elements can have surprising properties, even within the same group!
Are all metals good insulators?
Think of it like this: imagine a crowded room where people are constantly bumping into each other. This is similar to the way electrons in a metal behave. They’re free to move around and can easily transfer energy to other electrons.
Metals are so good at conducting electricity that they are commonly used in electrical wiring and appliances. They are also used in cookware and other applications where heat transfer is important.
On the other hand, insulators are materials that resist the flow of heat and electricity. They do this by having tightly bound electrons that are not free to move.
Some common examples of insulators include:
Rubber: This is often used to coat electrical wires to prevent shocks.
Glass: Used in windows and light bulbs because it prevents electricity from flowing through it.
Plastic: Commonly used for electrical plugs and casings because it doesn’t conduct electricity.
It’s important to remember that not all metals conduct electricity equally well. Some metals, like copper and silver, are very good conductors, while others, like iron and nickel, are less conductive.
This is because the number of free electrons and their ability to move freely vary between different metals. The more free electrons a metal has, the better it will conduct electricity.
So, while metals are generally good conductors, they are not good insulators. Their ability to conduct heat and electricity makes them crucial in many applications.
Are all metalloids insulators?
Think of semiconductors as a middle ground between conductors, which allow electricity to flow freely, and insulators, which block the flow of electricity. Metalloids can switch between these two roles depending on conditions like the presence of impurities or changes in temperature.
For example, silicon, a common metalloid, is a crucial component in many electronic devices. Under normal conditions, silicon behaves as an insulator. However, by introducing tiny amounts of impurities like phosphorus or boron, silicon can become a semiconductor. This allows silicon to control the flow of electricity, making it perfect for creating transistors and integrated circuits.
Imagine semiconductors as traffic lights for electrons. Sometimes they let the electrons flow freely (like a green light), and sometimes they stop the flow (like a red light). This ability to control the flow of electricity is what makes metalloids so valuable in the world of electronics.
So, while metalloids may not be perfect conductors, they have a remarkable ability to act as semiconductors. This versatility makes them essential for modern technology, allowing us to create everything from computers to solar panels.
See more here: Are All Non-Metals Insulators True Or False? | Are All Non Metals Insulators
What is a nonmetal insulator?
Think of it this way: electrons, those tiny particles carrying electrical charge, need a little push to start moving. In nonmetals, they have to overcome a barrier called the band gap. This band gap is like a tiny wall, and the amount of energy needed to jump over it determines whether a nonmetal is a good insulator or a semiconductor.
Insulators, like rubber and glass, have a big band gap. It takes a lot of energy to get electrons moving, so they resist electrical current very well. That’s why we use rubber gloves when working with electricity – it prevents us from getting shocked!
Semiconductors, like silicon, have a smaller band gap. They’re not as good at blocking electricity as insulators, but they’re not as conductive as metals either. This makes them perfect for building electronic components, like transistors and computer chips.
Now, here’s the interesting part: the higher the temperature, the easier it is for electrons to jump over the band gap. This means that nonmetals become slightly more conductive as they heat up. But even at high temperatures, they still act as pretty good insulators.
However, even the best insulator can break down under enough pressure, especially when exposed to high voltage and temperatures. This is called dielectric breakdown, and it happens when the electric field gets so strong that it forces electrons to jump across the band gap, creating a sudden flow of electricity. This can cause damage to the insulator and lead to electrical failures.
So, nonmetal insulators are super important for preventing the flow of electricity, and they’re crucial in many areas of our daily lives. Whether it’s protecting us from electric shocks, keeping our electronic devices functioning properly, or insulating our homes, nonmetal insulators play a vital role in our modern world.
Are insulators good conductors?
Let’s dive a little deeper into why this happens. The conductivity of a material depends on how easily electrons can move through it. In insulators, electrons are tightly bound to the atoms and don’t move freely. When you heat up an insulator, you give its atoms more energy. This extra energy can cause some electrons to break free from their atoms, making the material more conductive.
Think of it like a crowd of people. If everyone is standing still (like electrons in a cold insulator), it’s hard for anyone to move through. But if the crowd is excited and moving around (like electrons in a hot insulator), people can bump into each other and move more freely.
Metals, on the other hand, have free electrons that can easily move throughout the material. When you heat up a metal, the atoms vibrate more, making it harder for the electrons to move through the material. It’s like trying to walk through a crowded room—the more people are moving around, the harder it is to get through.
Superconductors are a fascinating exception. At extremely low temperatures, the electrons in some materials can form pairs and move together without any resistance. It’s like everyone in the crowd suddenly cooperating and moving in the same direction—no more bumping into each other! This phenomenon has the potential for revolutionizing energy transmission and other technologies.
What is an electrical insulator?
Think of it like this: Imagine a busy street with lots of people trying to get through. In a conductor, the people can move freely, representing electrons flowing easily through the material. In an insulator, though, the people are stuck in their places, representing the electrons that are tightly bound and can’t move.
Insulators are everywhere! You find them in your home, in your car, and even in your phone. For example, the plastic coating on your electrical wires is an insulator, keeping the electricity contained within the wire and preventing shocks. The rubber on your car’s tires acts as an insulator, preventing the flow of electricity from your car’s battery to the ground. And the glass in your phone screen is an insulator, keeping your fingers safe from the electrical components inside.
Let’s explore some common insulators in more detail:
Rubber: This is a very common insulator used in many applications, such as electrical wires, gloves for electricians, and tires.
Plastic: This is another popular insulator used for everything from electrical wires to water bottles.
Glass: This is an excellent insulator and is commonly used in light bulbs and windows.
Ceramic: This is a strong and durable insulator used in things like spark plugs and electrical components.
Wood: While not as good an insulator as the others, wood is still used in electrical applications, such as for electrical poles.
These are just a few examples of the many different insulators that exist. By understanding the role of insulators in electrical systems, we can better appreciate their importance in our everyday lives.
What makes a material a conductor or a insulator?
Simply put, conductors are materials that allow electricity to flow easily through them, while insulators resist the flow of electricity. The key difference lies in how easily electrons can move within these materials.
Imagine a bustling city street. People (electrons) can easily move around if there are wide, open sidewalks (conductors). But if the sidewalks are narrow and crowded (insulators), movement becomes difficult.
Conductors have a large number of free electrons that can easily move from atom to atom. These free electrons are like the people on a wide sidewalk – they can move easily, allowing electricity to flow. Metals like copper, silver, and gold are excellent conductors because they have many free electrons.
Insulators, on the other hand, have very few free electrons. Their electrons are tightly bound to their atoms, making it difficult for them to move. This is like having a narrow, crowded sidewalk – the people (electrons) have a hard time moving, preventing the flow of electricity. Materials like rubber, plastic, and glass are good insulators because their electrons are tightly bound.
The ability of a material to conduct electricity is measured by its conductivity. A high conductivity means the material is a good conductor, allowing electricity to flow easily. A low conductivity means the material is a good insulator, resisting the flow of electricity.
We often use conductors and insulators in everyday life. For instance, electrical wires are made from conductors like copper to carry electricity safely to our homes and appliances. The plastic coating around the wires acts as an insulator, preventing electric shocks.
So, the next time you see an electrical wire or a rubber-coated appliance, remember that these materials are carefully chosen based on their ability to either conduct or insulate electricity.
See more new information: countrymusicstop.com
Are All Nonmetals Insulators? The Surprising Truth
Nonmetals: The Insulators of the Periodic Table?
The periodic table is like a map of the elements, and nonmetals are a big part of the story. Think of them as the opposites of metals, those shiny, malleable materials that conduct electricity like champions. Nonmetals are usually dull, brittle, and…well, not very good conductors.
Why are nonmetals generally insulators? It all comes down to how their atoms are arranged and how their electrons behave. Metals have a “sea” of electrons that can move freely, allowing electricity to flow through them easily. Nonmetals, on the other hand, hold their electrons tightly. These electrons are not as free to move around, making it much harder for electricity to pass through.
Exceptions to the Rule: The Nonmetals that Conduct
Now, here’s where things get interesting. While it’s true that most nonmetals are excellent insulators, there are some exceptions.
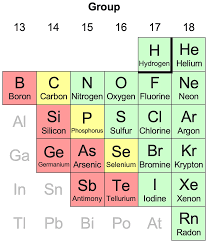
Graphite: This is a form of carbon, a quintessential nonmetal. But graphite has a unique structure where its electrons are free to move within its layers. This allows graphite to conduct electricity fairly well, making it a key ingredient in pencils and batteries.
Silicon: Silicon is a metalloid, a funky element that sits right on the border between metals and nonmetals on the periodic table. It’s a semiconductor, which means it can conduct electricity under certain conditions. Silicon is absolutely crucial for the modern world, forming the backbone of our computers, smartphones, and all kinds of electronic gadgets.
Phosphorus: This nonmetal can also conduct electricity under specific conditions. You can find phosphorus in things like fertilizers and detergents.
So, the rule that all nonmetals are insulators isn’t entirely accurate. There are a few exceptions, like graphite, silicon, and phosphorus, that can conduct electricity under certain circumstances.
Why do we care about insulators?
Think of insulators as the unsung heroes of the electrical world. They play a vital role in keeping us safe. Here’s why:
Safety:Insulators prevent us from getting shocked by electricity. You can see this in everyday objects like the plastic coating on electrical wires, the rubber handles on tools, and the porcelain parts of power lines. These insulators create a barrier between us and the live electrical components, keeping us safe.
Control:Insulators help us control the flow of electricity. They can be used to direct electricity where we want it to go, preventing short circuits and other electrical problems. Think about the insulating materials used in circuit boards, switches, and other electrical devices.
Efficiency: Insulators help reduce energy loss. By preventing electricity from escaping in unwanted directions, insulators ensure that the electrical energy reaches its destination efficiently. This is essential for everything from power plants to our homes and offices.
The Bottom Line: It’s a Matter of Conductivity
So, to answer the question, “Are all nonmetalsinsulators?” The answer is a bit more nuanced.
Most nonmetals are indeed insulators, thanks to the way their electrons are held. However, there are some notable exceptions, like graphite, silicon, and phosphorus, that can conduct electricity under specific conditions.
Understanding the role of insulators is essential in the world of electricity. They ensure our safety, control the flow of electrical energy, and contribute to its efficient use.
FAQs
Here are a few questions you might have about nonmetals and insulators:
1. Are all metals conductors?
Yes, most metals are good conductors of electricity. This is because they have a “sea” of loosely bound electrons that can move freely, allowing electricity to flow through them easily.
2. What are some common examples of insulators?
Here are some common examples:
Plastic: You see this everywhere, from the coating on wires to your smartphone cases.
Rubber: Think about the handles on tools and the soles of shoes.
Glass: Commonly found in electrical components and lightbulbs.
Wood: A good insulator, used in many applications, from building materials to furniture.
Air: Surprisingly, air is a pretty good insulator too.
3. What makes a good insulator?
A good insulator has a large band gap, which means its electrons are tightly bound and resistant to moving. This means that electricity has a hard time flowing through it.
4. Can you give me some examples of how insulators are used in our daily lives?
Absolutely! Here are a few:
Electrical Wiring: The plastic coating on wires insulates the live wires from the outside world, keeping us safe.
Circuit Boards: Insulators help direct the flow of electricity within electronic circuits.
Power Lines: The porcelain insulators on power lines help prevent electricity from escaping to the ground.
Appliances: The plastic casings on appliances like ovens and refrigerators act as insulators to protect us from electrical shocks.
5. What are some other interesting facts about nonmetals?
Here are a few:
Nonmetals can exist in all three states of matter: solid, liquid, and gas.
Nonmetals are essential for life. For example, oxygen and carbon are crucial for breathing and forming the basis of organic molecules.
Nonmetals are used in various industries, including pharmaceuticals, electronics, and agriculture.
6. Can nonmetals become conductors?
Yes, certain nonmetals can become conductors under specific conditions. For example:
Graphite becomes a good conductor when it is compressed or heated.
Silicon can become a conductor when it is doped with other elements, making it a semiconductor.
Remember, the world of electricity is full of fascinating things. Understanding the differences between conductors and insulators, and how nonmetals play a role in this, is a great way to appreciate the technology that shapes our modern world.
10 Examples of Electrical Conductors and Insulators
Simply put, electrical conductors are materials that carry (or conduct) electrical currents well, such as iron and steel, and insulators ThoughtCo
Nonmetal | Definition, Properties, Examples, & Facts
From a chemical point of view, nonmetals may be divided into two classes: 1) covalent materials, which contain atoms having small Britannica
Electricity – Conductors, insulators, and semiconductors
Electricity – Conductors, insulators, and semiconductors: Materials are classified as conductors, insulators, or semiconductors according to their electric conductivity. The classifications can be Britannica
Conductors and Insulators – HyperPhysics
In a conductor, electric current can flow freely, in an insulator it cannot. Metals such as copper typify conductors, while most non-metallic solids are said to be good insulators, HyperPhysics
Choosing and using conductors and insulators (non-statutory)
Only metals are electrical conductors. All non-metals are electrical insulators. Children will be taught some non-metals are poor insulators, e.g. water as it can contain Oak National Academy
Physics Tutorial: Conductors and Insulators
Examples of insulators include plastics, Styrofoam, paper, rubber, glass and dry air. The division of materials into the categories of conductors and insulators is a somewhat artificial division. It is more appropriate to The Physics Classroom
7.3: Conductors and Insulators – Physics LibreTexts
These are called insulators. Electrons and ions in insulators are bound in the structure and cannot move easily—as much as 1023 10 23 times more slowly than in Physics LibreTexts
Lesson: Electrical conductors and insulators | KS2 Science | Oak …
Only metals are electrical conductors. All non-metals are electrical insulators. Water is an electrical conductor. Children will test materials for conductivity, but the teaching slides Oak National Academy
Conductors and insulators (video) | Khan Academy
Conductors and insulators. Google Classroom. About Transcript. A conductor is a material that allows electrons to flow freely through it, making it useful for carrying Khan Academy
What Are Conductors \U0026 Insulators? Are All Metals Conductors? Are All Non-Metals Insulators? (Nso)
Conductors \U0026 Non-Conductors | Properties Of Matter | Chemistry | Fuseschool
Conductors And Insulators – Examples, Definition, Properties | Video For Kids
Form 2 | Science Pt3| Heat Conductors And Heat Insulators
Electrical Conductors And Insulators
Link to this article: are all non metals insulators.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
