Is C60 fullerene sp2?
C60 fullerene is a fascinating molecule, often referred to as a “buckyball” due to its resemblance to a soccer ball. It’s made up of 60 carbon atoms arranged in a spherical shape. Now, to understand sp2 hybridization, we need to look at the way carbon atoms bond.
You’re probably familiar with the idea that carbon has four valence electrons, which means it can form four bonds. In sp2 hybridization, one s orbital and two p orbitals mix to form three sp2 hybrid orbitals, while the remaining p orbital stays pure. These sp2 hybrid orbitals are arranged in a trigonal planar geometry, forming strong bonds with other atoms.
The average poavI a-bond hybridization for C60 is sp2.278. This means that the bonds in C60 are not perfectly sp2 hybridized, but rather a blend of sp2 and sp3. The presence of sp3 character leads to some distortion in the ideal sp2 geometry.
It’s also important to understand the role of the rc-orbital fractional s-character. This refers to the percentage of s character in the remaining p orbital, which isn’t involved in the sp2 hybridization. For C60, this s-character is found to be 0.085 (poavI) and 0.081 (poav2). This indicates that the p orbital in C60 has a slight s character, contributing to the unique bonding properties of this molecule.
The sp2.278 hybridization and the s-character in the remaining p orbital are important factors that contribute to the stability and unique properties of C60 fullerene. This slightly distorted sp2 hybridization is responsible for the molecule’s remarkable strength and resilience.
To illustrate this further, let’s think about the iconic soccer ball shape of C60. Imagine each carbon atom at the vertices of the ball. Each carbon atom forms three bonds with its neighbors, creating a network of interconnected hexagons and pentagons. While these bonds might not be perfectly sp2 hybridized, they are still strong and rigid, contributing to the overall stability of the structure.
So, while C60 fullerene is not perfectly sp2 hybridized, its unique bonding pattern with a mix of sp2 and sp3 character makes it a fascinating example of molecular structure and stability.
What is the hybridization of the structure C60?
C60, also known as buckminsterfullerene, is a molecule composed of 60 carbon atoms arranged in a spherical shape. Each carbon atom is bonded to three other carbon atoms, forming a network of interconnected pentagons and hexagons. This structure resembles a soccer ball, hence the nickname buckyball.
sp2 hybridization involves the mixing of one s orbital and two p orbitals from each carbon atom. This mixing results in three sp2 hybrid orbitals that are arranged in a trigonal planar geometry. Each sp2 hybrid orbital forms a sigma bond with another carbon atom, while the remaining unhybridized p orbital forms a pi bond with the adjacent carbon atom. These pi bonds contribute to the overall stability and unique electronic properties of C60.
The sp2 hybridization model provides a simplified but effective way to understand the bonding and electronic structure of C60. It helps explain the geometry of the molecule, the strength of the carbon-carbon bonds, and the existence of pi electrons that contribute to the molecule’s unique properties. While this model is a good starting point, more complex models are needed to fully understand the intricate details of the C60 molecule.
Is graphite sp2 or Sp3?
Let’s break this down. Carbon has four valence electrons, which are located in the s and p orbitals. In graphite, one s orbital combines with two p orbitals to form three sp2 hybrid orbitals. These sp2 hybrid orbitals are arranged in a trigonal planar geometry with bond angles of 120 degrees. The remaining p orbital remains unhybridized and forms a pi bond with an adjacent carbon atom.
These sp2 hybrid orbitals are responsible for the strong, planar, hexagonal rings that form the layers of graphite. These layers are held together by weak van der Waals forces, which allow them to slide past each other easily. This is what gives graphite its characteristic lubricating properties. The unhybridized p orbital forms a pi bond with an adjacent carbon atom, creating a delocalized electron system. This is why graphite is a good conductor of electricity.
Here’s a breakdown of why graphite is sp2 hybridized:
Formation of sp2 hybrid orbitals: One s orbital and two p orbitals combine to form three sp2 hybrid orbitals.
Trigonal Planar Geometry: The sp2 hybrid orbitals are arranged in a trigonal planar geometry, with bond angles of 120 degrees, forming the planar, hexagonal rings.
Delocalized Electrons: The unhybridized p orbitals form pi bonds, resulting in a delocalized electron system. This is what makes graphite a good conductor of electricity.
Understanding the hybridization of carbon atoms in graphite is key to understanding its properties. This sp2 hybridization plays a crucial role in the structure, properties, and applications of this versatile material.
Is C60 a sp3?
All carbons in C60 are identical and have sp2 hybridization. This means each carbon atom forms three sigma bonds with its neighboring carbon atoms, resulting in a planar hexagonal or pentagonal structure. The remaining electron on each carbon atom participates in a delocalized pi system that extends over the entire molecule. This delocalization of electrons contributes to the exceptional stability of C60.
Let’s break down why C60 doesn’t have sp3 hybridization. sp3 hybridization occurs when a carbon atom forms four sigma bonds, resulting in a tetrahedral geometry. In C60, if the carbon atoms were sp3 hybridized, the molecule would have a three-dimensional, cage-like structure with a different number of carbon atoms. Imagine a soccer ball – C60 resembles this shape because of the sp2 hybridization of its carbon atoms. The sp2 hybridization allows for a planar, two-dimensional arrangement of the carbon atoms, creating the unique spherical shape of C60.
Think of it this way: sp2 hybridization is like a flat sheet of paper, while sp3 hybridization is like a three-dimensional cube. C60 is formed by connecting several “sheets” of sp2 hybridized carbon atoms to create its unique spherical structure.
Is carbon 60 C60 or C60 fullerenes?
C60 is actually a type of fullerene. It’s the most common and well-studied fullerene, and it’s what people usually mean when they just say “fullerene.” Imagine a hollow soccer ball made entirely of carbon atoms – that’s C60 in a nutshell. It’s a fascinating structure, with 60 carbon atoms forming a sphere with 20 hexagons and 12 pentagons.
Fullerenes are a class of carbon molecules that have a closed, cage-like structure. Think of them like soccer balls but with various shapes and sizes, all built from carbon atoms. C60, with its iconic soccer ball shape, is just one member of this family.
Now, why are fullerenes, especially C60, so special? The unique arrangement of carbon atoms gives them some amazing properties, like strong antioxidant activity. This is because the C60 molecule can interact with free radicals in the environment, helping to neutralize their damaging effects.
Fullerenes and C60 are an exciting area of research, with potential applications in fields like medicine, materials science, and energy. So next time you hear about C60, remember – it’s a fascinating molecule with a lot of potential, and it’s just one member of the broader fullerene family.
What hybridization is fullerene?
Each carbon atom in a fullerene molecule forms three sigma bonds with its neighboring carbon atoms. These bonds are formed by the overlap of one sp2 hybrid orbital from each carbon atom. The remaining p orbital on each carbon atom is not involved in the sigma bonding and overlaps with the p orbitals of adjacent carbon atoms, forming a delocalized pi system. This pi system extends over the entire fullerene molecule, giving it its unique properties.
Think of it like this: Imagine a flat sheet of paper. Each carbon atom is like a point on that sheet, and the sigma bonds are like the lines connecting those points. The pi system is like a thin layer of glue that holds the whole sheet together. In a fullerene, this sheet of paper is curved into a sphere or an ellipsoid, giving the molecule its characteristic three-dimensional structure.
Fullerenes are known for their high thermal stability and electrical conductivity, both of which are attributed to the presence of the sp2 hybridization and the delocalized pi system. This is why they have found applications in various fields, such as materials science, medicine, and electronics.
How do you determine carbon hybridization?
A handy trick to figure out hybridization is to count the number of atoms bonded to the carbon atom and the number of lone pairs. Remember, double and triple bonds still count as being only bonded to one atom.
For example, let’s say a carbon atom is bonded to two other atoms. This means it needs two hybrid orbitals, resulting in an sp hybridization.
Now, let’s explore this further:
sp Hybridization: When a carbon atom forms two sigma bonds and no lone pairs, it undergoes sp hybridization. The carbon atom uses one *s* orbital and one *p* orbital to create two sp hybrid orbitals. These hybrid orbitals are oriented at an angle of 180 degrees, giving rise to a linear molecular geometry.
sp² Hybridization: When a carbon atom forms three sigma bonds and no lone pairs, it undergoes sp² hybridization. The carbon atom uses one *s* orbital and two *p* orbitals to form three sp² hybrid orbitals. These orbitals are arranged in a trigonal planar geometry with bond angles of 120 degrees.
sp³ Hybridization: When a carbon atom forms four sigma bonds or three sigma bonds and one lone pair, it undergoes sp³ hybridization. The carbon atom uses one *s* orbital and three *p* orbitals to create four sp³ hybrid orbitals, which are arranged in a tetrahedral geometry with bond angles of 109.5 degrees.
To summarize, understanding the number of atoms bonded to the carbon atom and lone pairs allows us to determine the hybridization of a carbon atom. This knowledge is crucial for predicting the molecular geometry and bond angles of organic molecules.
What is the structure of C60 fullerene?
But what makes this structure so special? The combination of pentagons and hexagons is what gives C60 its strength and stability. If you were to build a sphere only from hexagons, you would end up with a flat sheet. It’s the pentagons that introduce curvature, allowing the molecule to form a closed, three-dimensional shape. Imagine trying to make a ball out of only flat pieces of paper – you need some curved pieces to create a sphere.
The pentagons also play a crucial role in the stability of the fullerene structure. Each carbon atom in C60 is bonded to three other carbon atoms, forming a network of strong, covalent bonds. These bonds are all equally spaced and arranged in a way that minimizes the energy of the molecule, making it highly stable.
In fact, the structure of C60 fullerene is so stable that it can withstand high temperatures, pressures, and even chemical reactions without breaking down. This stability is one of the reasons why C60 fullerenes have become so popular for use in a variety of applications, including medicine, electronics, and materials science.
See more here: What Is The Hybridization Of The Structure C60? | Hybridization Of Carbon In Fullerene
Is fullerene a variable or intermediate hybridization?
Fullerenes, like C60, have a curved surface. This curvature means their hybridization lies somewhere between sp2 (found in graphite) and sp3 (found in diamond). This means that the carbon atoms in fullerenes exhibit an intermediate hybridization.
But it gets a bit more complex. The POAV1 theory suggests that the carbon atoms in C60 have an sp2.28 hybridization. This isn’t a perfect sp2 or sp3, but rather a unique blend. This indicates that the hybridization in fullerenes might be variable, depending on the specific fullerene structure and its curvature.
Think of it this way: imagine a spectrum of hybridization, with sp2 on one end and sp3 on the other. Fullerenes exist somewhere in the middle of this spectrum, and their precise hybridization can vary depending on their structure.
The fact that fullerenes have variable hybridization is one reason why they are so intriguing and offer a wide range of potential applications. This unique characteristic contributes to their diverse electronic and optical properties, making them promising materials for electronics, medicine, and other fields.
Are fullerenes stable?
But don’t worry, this angle strain doesn’t make fullerenes crumble! The sp2 hybridization allows for a certain amount of flexibility, and the strong carbon-carbon bonds help hold everything together. Think of it like a flexible, but strong, mesh structure.
Now, this angle strain does have some interesting consequences. It makes fullerenes a bit more reactive than their planar counterparts like graphite. They can undergo reactions that involve the addition of atoms or molecules to the fullerene cage, or even the opening of the cage to form different structures.
For instance, fullerenes can react with other molecules to form adducts, which are essentially molecules that have been joined together. These reactions can happen through a process called cycloaddition, where a molecule adds to a double bond in the fullerene cage. Think of it like attaching a piece of string to a mesh bag.
Overall, fullerenes are remarkably stable molecules, considering the strain they have to endure to form those unique shapes. They might be a bit more reactive than their flat cousins, but that just adds to their fascinating chemistry!
How many carbon atoms are in a fullerene?
Let’s explore the range of carbon atoms that can be found in fullerenes. The most famous fullerene, buckminsterfullerene (also known as C60), contains 60 carbon atoms. However, you can find fullerenes with different numbers of carbon atoms, ranging from a minimum of 28 to a maximum of 1500. The specific number of carbon atoms in a fullerene determines its properties and potential applications.
For instance, the C76, C80, and C240 fullerenes are larger than C60 and are synthesized using a greater number of carbon atoms. This implies that the larger the fullerene, the greater the number of carbon atoms needed to form its structure. Imagine these fullerenes as intricate, spherical cages made of carbon atoms, with each cage having a unique size and shape depending on the number of carbon atoms involved.
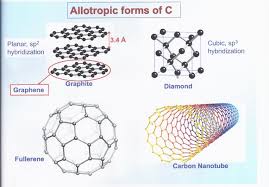
What is the hybridization state of graphite and Diamond?
Graphite has a sp2 hybridization state. This means each carbon atom forms three sigma bonds, resulting in a flat, sheet-like structure. These sheets are then held together by weak van der Waals forces, which is why graphite is soft and slippery.
In diamond, the carbon atoms are in a sp3 hybridization state. Each carbon forms four sigma bonds, creating a rigid, three-dimensional network. This strong, tetrahedral arrangement is what makes diamond the hardest naturally occurring material.
Now, let’s talk about fullerenes, which are another fascinating form of carbon. Fullerenes have a closed, cage-like structure. The surface of a fullerene is not perfectly planar like graphite, and the hybridization of the carbon atoms on the surface is somewhere between sp2 and sp3. This “in-between” state is due to the curvature of the fullerene structure.
In simpler terms:
Graphite: Imagine a flat sheet of paper where each carbon atom is connected to three neighbors. The sheets are stacked on top of each other, but they can easily slide past each other, giving graphite its slippery texture.
Diamond: Picture a three-dimensional network of carbon atoms where each atom is connected to four neighbors. This strong network is what gives diamond its exceptional hardness.
Fullerenes: Think of a soccer ball, but with each point representing a carbon atom. The carbon atoms on the surface of the ball are arranged in a slightly more complex way than in graphite or diamond, giving them a hybridization state somewhere between sp2 and sp3.
So, to summarize:
Graphite:sp2 hybridization, flat sheet-like structure
Diamond:sp3 hybridization, rigid three-dimensional network
Fullerenes: Hybridisation between sp2 and sp3, closed, cage-like structure
The different hybridization states of carbon are responsible for the unique properties of these incredible materials.
See more new information: countrymusicstop.com
Hybridization Of Carbon In Fullerene: A Deep Dive
Buckminsterfullerene, affectionately known as buckyball, is a fascinating molecule. It’s a spherical cage made entirely of carbon atoms, and it’s a fantastic example of hybridization in action. But before we dive into the specifics of fullerene hybridization, let’s quickly review what hybridization means.
Hybridization is the concept of mixing atomic orbitals to create new hybrid orbitals. These hybrid orbitals have different shapes and energies than the original orbitals, which allows them to form stronger bonds with other atoms. This is crucial in understanding the structure of fullerenes.
Understanding Carbon Hybridization in Fullerenes
Each carbon atom in a fullerene molecule forms three sigma bonds (single bonds) and one pi bond (double bond). This bonding pattern is unique and requires a specific type of hybridization: sp2 hybridization.
Here’s how it works:
1. Mixing Orbitals: A carbon atom has one 2s orbital and three 2p orbitals. These orbitals combine to form three sp2 hybrid orbitals and one remaining p orbital.
2. Sp2 Hybridization and Geometry: The three sp2 orbitals are arranged in a trigonal planar geometry. This means they point outwards from the carbon atom at 120° angles to each other, forming the familiar planar hexagonal rings found in fullerenes.
3. Pi Bonding: The remaining p orbital on each carbon atom overlaps with the p orbitals of its neighboring atoms to form pi bonds. These pi bonds are delocalized over the entire fullerene structure, giving it its unique aromatic character.
Why sp2 Hybridization?
The sp2 hybridization of carbon in fullerenes is critical for the stability and unique properties of these molecules. Here’s why:
Stronger Bonds: The sigma bonds formed by the sp2 orbitals are stronger than the bonds that would be formed by using the original s and p orbitals. This contributes to the overall stability of the fullerene molecule.
Planar Structure: The trigonal planar geometry of the sp2 hybrid orbitals allows for the formation of the hexagonal rings that make up the fullerene cage.
Aromatic Character: The delocalized pi system created by the overlap of p orbitals gives fullerenes their distinctive aromatic character. This contributes to their unique chemical and physical properties, like their exceptional stability, conductivity, and reactivity.
The Importance of Hybridization in Fullerenes
The hybridization of carbon in fullerenes is a critical factor in understanding their behavior. Without it, we wouldn’t have the unique structures, properties, and potential applications that have made these fascinating molecules so exciting.
Let’s explore some of these applications:
Electronics: Fullerenes are promising materials for organic electronics. Their high conductivity and ability to absorb light make them ideal for use in solar cells, transistors, and other electronic devices.
Medicine: The unique structure of fullerenes allows them to interact with biological systems in unique ways. This has led to research into their potential uses in drug delivery, gene therapy, and even as anti-cancer agents.
Materials Science: The strength and stability of fullerenes make them attractive for use in materials science. They can be used to reinforce polymers, create new composites, and even develop ultra-thin films for various applications.
FAQs on Hybridization in Fullerenes
Now, let’s dive into some of the common questions about hybridization in fullerenes:
1. Why don’t fullerenes have sp3 hybridization like diamond?
Fullerenes have a different bonding structure than diamond. While diamond forms a continuous network of interconnected tetrahedral carbon atoms (sp3 hybridization), fullerenes are closed cage structures with hexagonal and pentagonal rings. The formation of these rings requires sp2 hybridization for the carbon atoms.
2. How does the delocalized pi system in fullerenes affect their properties?
The delocalized pi system contributes to the unique properties of fullerenes:
Stability: The delocalization of electrons strengthens the overall molecule.
Aromaticity: The delocalized pi system is characteristic of aromatic compounds, leading to unique chemical reactivity and stability.
Conductivity: The free movement of electrons in the pi system contributes to the electrical conductivity of fullerenes.
3. Can fullerenes exist with different hybridization?
Yes, there are fullerenes with different hybridizations. For instance, the fullerene nanotube exhibits both sp2 and sp3 hybridization, where the tube’s cylindrical structure involves sp2 hybridization, and the cap regions can include sp3 hybridization.
4. What are the limitations of fullerenes?
While fullerenes hold great potential, there are limitations to consider:
Toxicity: Some fullerenes can be toxic to living organisms.
Production: Large-scale production of high-purity fullerenes can be challenging.
Stability: While stable, fullerenes can undergo reactions under certain conditions.
5. What is the future of fullerene research?
The field of fullerene research is constantly evolving. Ongoing research focuses on:
Synthesizing new fullerenes: Scientists are exploring new methods to synthesize fullerenes with different sizes and structures.
Understanding their properties: Research continues to delve into the unique properties of fullerenes and their potential applications.
Developing new applications: Researchers are constantly exploring new uses for fullerenes in electronics, medicine, materials science, and other fields.
Final Thoughts
Understanding the hybridization of carbon in fullerenes is essential for appreciating their unique properties and potential applications. It’s a fascinating example of how the simple concept of atomic orbital mixing can lead to incredibly complex and useful molecules. This field is constantly evolving, and who knows what amazing breakthroughs await in the future of fullerene research!
Structural Characteristics of Fullerenes | SpringerLink
By comparison, the hybridization of all the carbon atoms on the fullerene surface is between sp 2 and sp 3, and sometimes approximately sp 2 though the surface is generally not as planar as that of graphite. Each carbon atom forms three carbon Springer
The fullerenes: powerful carbon-based electron acceptors …
Because of the curvature of the surface, fullerene hybridization falls between graphite (sp 2) and diamond (sp 3) and these new carbon allotropes are therefore of intermediate, and perhaps variable hybridization. According to POAV1 theory the carbon royalsocietypublishing.org
Development of Fullerenes and Their Derivatives | SpringerLink
C 60 fullerenes are highly reactive toward halogens due to the presence of electron-rich sp 2-hybridized carbon atoms on their surface, which contain delocalized π-electrons. This reactivity allows fullerenes to undergo halogenation reactions, where a Springer
Structures and Stability of Fullerenes, Metallofullerenes, and Their …
This chapter describes general principles in the stability and bonding of empty fullerenes, endohedral fullerenes, and exohedral derivatives of empty fullerenes. First, an overview of the structural properties of empty fullerenes is given. Springer
Allotropes Of Carbon – Graphite, Diamond, Graphene, \U0026 Fullerenes
Gcse Chemistry – Allotropes – Graphene And Fullerenes #19
The Hybridisation State Of Carbon In Fullerene Is (A) S(B) S P^2 (C) S P^3 (D) S P^3 D
Bucky Balls, Nanotubes \U0026 Graphene | Organic Chemistry | Chemistry | Fuseschool
Hybridization Of Atomic Orbitals – Sigma \U0026 Pi Bonds – Sp Sp2 Sp3
Link to this article: hybridization of carbon in fullerene.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
