What does Br2 do to carboxylic acid?
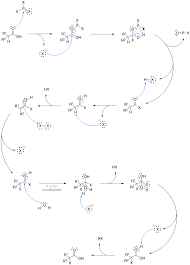
You’re right to focus on the reaction with PBr3 first. This is the key to getting things started. PBr3 converts the carboxylic acid into an acid bromide. Think of it as replacing the -OH group of the acid with a -Br. This reaction also generates HBr, which is a crucial player in the next step.
Now, the HBr catalyzes the formation of an enol from the acid bromide. Enols are special molecules that can switch between a ketone and an alcohol form. This shift is important because it opens up a new site for bromine to react.
The enol, now ready for action, reacts with Br2. This is where the α-substitution happens. The bromine atom adds to the carbon next to the carbonyl group (the α-carbon). This gives us an α-bromo acid bromide as the final product.
Let’s dive a bit deeper into the process.
Why does HBr catalyze enolization? HBr is a strong acid, and it can protonate the carbonyl oxygen of the acid bromide. This makes the carbonyl group more electrophilic (electron-loving), making it easier for the alpha hydrogen to be removed by a base. Think of it like making the carbonyl group a magnet for the alpha hydrogen. This leads to the formation of the enol.
Why does the enol react with Br2? The enol has a double bond, which makes it susceptible to attack by the electrophilic bromine. The bromine adds to the carbon atom, which is attached to the hydroxyl group of the enol, forming the α-bromo acid bromide.
What’s the significance of α-substitution? This type of reaction is important in organic chemistry because it allows us to introduce a halogen atom at a specific position on a molecule. This can be used to synthesize other important compounds.
Think of it like building with Lego blocks. You start with a basic carboxylic acid, then you use the right tools (PBr3 and HBr) to modify it into a new structure. This new structure, the α-bromo acid bromide, opens up a world of possibilities for further reactions and creating new molecules.
Which of the following acids does not undergo the Hell Volhard Zelinsky reaction?
The HVZ reaction is a way to convert a carboxylic acid to an $\alpha$-halo carboxylic acid. It involves the reaction of a carboxylic acid with bromine and phosphorus tribromide.
The key to the reaction is the presence of an $\alpha$-hydrogen atom. 2,2-dimethylpropanoic acid lacks this critical $\alpha$-hydrogen atom because its $\alpha$ carbon is completely substituted with methyl groups. Without an $\alpha$-hydrogen, the HVZ reaction cannot occur.
Let’s break it down:
Carboxylic Acids: These organic compounds contain a carboxyl functional group (-COOH).
$\alpha$-hydrogen: The hydrogen atom attached to the carbon atom next to the carboxyl group is called the $\alpha$-hydrogen. It’s this hydrogen that gets replaced in the HVZ reaction.
2,2-dimethylpropanoic acid: This specific acid has two methyl groups attached to the carbon next to the carboxyl group. Since this carbon is already fully substituted, there’s no room for an $\alpha$-hydrogen.
Think of it like a puzzle piece. The HVZ reaction needs that $\alpha$-hydrogen to fit into its mechanism. Since 2,2-dimethylpropanoic acid doesn’t have that piece, it can’t participate in the reaction.
This is why 2,2-dimethylpropanoic acid is the only option that doesn’t undergo the HVZ reaction.
What does PBr3 and Br2 do to a carboxylic acid?
First, the carboxylic acid reacts with PBr3, forming the acid bromide and HBr. This HBr then acts as a catalyst, promoting the formation of the acid bromide enol. This enol is a special form of the acid bromide where a hydrogen atom on the carbon next to the carbonyl group is replaced by a hydroxyl group. This change in structure opens up a new possibility for reaction.
The acid bromide enol then reacts with Br2, leading to alpha bromination. In this step, a bromine atom attaches to the carbon adjacent to the carbonyl group, creating an alpha-bromo acid bromide. This bromination step is essential for many synthetic reactions.
Finally, the acid bromide reacts with water, reforming the carboxylic acid. This step is crucial for completing the overall transformation.
Here’s a deeper dive into each step:
Step 1: Formation of the Acid Bromide
PBr3 is a powerful reagent that reacts with carboxylic acids to replace the hydroxyl group (-OH) with a bromine atom (-Br).
* This reaction is usually carried out in the presence of a solvent like dichloromethane or ether.
* The reaction proceeds through an intermediate known as an acyl bromide.
* This acyl bromide is a highly reactive compound that is readily converted to the acid bromide upon further reaction with PBr3.
* The HBr that is formed during this process is a key catalyst for the subsequent reactions.
Step 2: Formation of the Acid Bromide Enol
HBr acts as a catalyst, promoting the formation of the acid bromide enol.
* This step involves the removal of a proton from the carbon atom adjacent to the carbonyl group (the alpha carbon).
* The resulting negative charge on the alpha carbon is then delocalized to the oxygen atom of the carbonyl group, leading to the formation of a enol structure.
* The enol form is a tautomer of the acid bromide, which means that it is an isomer that can readily convert back to the acid bromide form.
Step 3: Alpha Bromination
* The acid bromide enol is very reactive, especially towards electrophiles such as bromine molecules.
Br2 readily reacts with the enol form of the acid bromide, leading to the addition of a bromine atom at the alpha position.
* This reaction is highly regioselective, meaning that the bromine atom is preferentially added to the alpha carbon.
* The addition of bromine at the alpha position creates an alpha-bromo acid bromide structure.
Step 4: Hydrolysis
* The acid bromide is a highly reactive compound and is easily hydrolyzed by water.
* The hydrolysis reaction results in the reformation of the carboxylic acid.
* The reaction proceeds through the addition of water to the carbonyl group, followed by the elimination of HBr.
* This hydrolysis step is typically carried out in the presence of a base such as sodium hydroxide.
By understanding these steps, you can appreciate how PBr3 and Br2 work together to achieve a specific transformation of carboxylic acids. This reaction is widely used in organic chemistry to synthesize a variety of important compounds.
Which carboxylic acid reacts with Br2 and red phosphorus?
The key to understanding this reaction lies in the concept of alpha-hydrogens. These are hydrogen atoms attached to the carbon atom adjacent to the carboxyl group (-COOH) in a carboxylic acid.
The reaction you’re asking about is called the Hell-Volhard-Zelinsky (HVZ) reaction. It’s a powerful tool for introducing a bromine atom at the alpha position of a carboxylic acid.
Here’s how it works:
1. Activation: The first step involves the reaction of red phosphorus with bromine to form phosphorus tribromide (PBr3). This compound acts as an activating agent, promoting the reaction with the carboxylic acid.
2. Formation of the Alpha-Bromo Acid: The phosphorus tribromide then reacts with the carboxylic acid, converting it into an acyl bromide intermediate. This intermediate is highly reactive and readily undergoes bromination at the alpha position due to the presence of alpha-hydrogens.
3. Hydrolysis: The final step involves hydrolysis, which converts the acyl bromide back into the alpha-bromocarboxylic acid.
The HVZ reaction is a valuable tool in organic synthesis for introducing bromine at a specific position in a molecule. It’s used in the synthesis of various important compounds, including pharmaceuticals and agricultural chemicals.
In short, the key point to remember is that only carboxylic acids with alpha-hydrogens can undergo the HVZ reaction to form alpha-bromocarboxylic acids.
Now, let’s look at the reaction in more detail:
Why is red phosphorus used?
Red phosphorus is a crucial component in the HVZ reaction. It’s used to generate phosphorus tribromide (PBr3) from bromine. This reaction is exothermic and releases heat. Red phosphorus serves as a catalyst, accelerating the reaction and ensuring a smooth conversion of bromine to phosphorus tribromide.
Why is bromine used?
Bromine is a powerful electrophile that readily reacts with the alpha-carbon in the carboxylic acid. It’s responsible for introducing the bromine atom at the alpha position.
What about the alpha-hydrogens?
The presence of alpha-hydrogens is critical for the reaction to occur. These hydrogens are acidic due to the electron-withdrawing effect of the carboxyl group. The acidity of these hydrogens makes them susceptible to abstraction by the phosphorus tribromide, creating a reactive intermediate that can then react with bromine.
The HVZ reaction is a classic example of how understanding the structure and reactivity of organic molecules can lead to powerful synthetic strategies.
What does Br2 do to an alkyne?
Br2 is a halogen and alkynes are hydrocarbons with a triple bond. When Br2 approaches an alkyne, the triple bond acts as a nucleophile, meaning it attracts the positive end of the Br2 molecule. This attraction causes the Br2 molecule to become polarized, with one bromine atom becoming slightly positive and the other slightly negative. The π electrons in the triple bond then attack the slightly positive bromine atom, forming a C-Br bond and kicking off a bromide ion. This is an electrophilic addition reaction.
This reaction can happen twice because alkynes have two π bonds. The first reaction forms a dibromoalkene. A second Br2 molecule can then add to the dibromoalkene to form a tetrabromoalkane.
Here’s a closer look at the reaction:
Step 1: The Br2 molecule approaches the alkyne.
Step 2: The π electrons in the triple bond attack the slightly positive bromine atom.
Step 3: A C-Br bond forms, and a bromide ion is released.
Step 4: The reaction repeats, adding another bromine atom to the other side of the triple bond.
Important Notes:
* The reaction is stereospecific, meaning the bromine atoms add to the same side of the alkyne.
* The reaction is exothermic, meaning it releases heat.
Br2 is a strong electrophile, which means it is easily attracted to electron-rich molecules like alkynes.
This reaction is very useful in organic chemistry, as it can be used to synthesize a variety of dibromoalkenes and tetrabromoalkanes. These compounds are important intermediates in many organic reactions.
Let me know if you’d like to delve into more details about these intermediates or any other aspect of this reaction!
Why doesn’t formic acid undergo the HVZ reaction?
Formic acid, however, is a bit of an oddball. It has only one carbon atom, and it’s directly bonded to the carboxyl group. This means formic acid doesn’t have an alpha carbon, and therefore, no alpha hydrogen to work with. That’s why formic acid can’t participate in the HVZ reaction.
Think of it this way: Imagine you want to build a house, and you need a certain type of brick. The HVZ reaction is like the builder, and the alpha hydrogen is the brick. Formic acid is like a house with no foundation; there’s no place for the brick to go, so the builder can’t do its job.
To put it another way, the HVZ reaction needs a specific piece of the molecule to work. It’s like trying to fit a square peg in a round hole; it just won’t fit. Formic acid, lacking the required piece, can’t take part in the HVZ reaction.
Why is carboxylic acid stronger than phenol?
When a carboxylic acid loses a proton (H+), it forms a carboxylate ion. The carboxylate ion is incredibly stable because the negative charge can be delocalized across both oxygen atoms through resonance. This means the negative charge isn’t concentrated on a single atom, making the ion more stable.
Now, let’s look at phenols. When a phenol loses a proton, it forms a phenoxide ion. In the phenoxide ion, the negative charge is mainly localized on the oxygen atom. However, it can also be delocalized slightly into the benzene ring through resonance. However, this delocalization is less effective since the carbon atoms in the ring are less electronegative than oxygen. As a result, the phenoxide ion is less stable compared to the carboxylate ion.
Think of it this way: The carboxylate ion is like spreading the negative charge across two oxygen atoms, while the phenoxide ion is like trying to spread it across a larger, less electronegative area. This makes the carboxylate ion much more comfortable and stable, leading to a stronger acid.
In essence, the ability of the carboxylate ion to spread out the negative charge through resonance more effectively than the phenoxide ion is the key reason why carboxylic acids are stronger acids than phenols.
See more here: What Does Br2 Do To Carboxylic Acid? | Hell Volhard Zelinsky Reaction Amino Acid
Who created the Hell-Volhard-Zelinsky reaction?
Let’s take a closer look at the contributions of each chemist:
Carl Magnus von Hell (1849–1926) was a German chemist known for his work in organic chemistry. He made significant contributions to the understanding of the structure and reactions of organic compounds. Hell’s research on halogenation reactions of carboxylic acids laid the groundwork for the later development of the Hell-Volhard-Zelinsky reaction.
Jacob Volhard (1834–1910) was another prominent German chemist who focused on organic synthesis. He made key advancements in the field of analytical chemistry and developed a method for determining the amount of silver in a solution. Volhard’s work on the halogenation of carboxylic acids, building upon Hell’s research, led to the development of the Hell-Volhard-Zelinsky reaction.
Nikolay Zelinsky (1861–1953) was a Russian chemist renowned for his contributions to organic chemistry, particularly in the field of catalysis. Zelinsky’s research focused on the use of platinum as a catalyst for various chemical reactions. He also conducted extensive studies on the chemistry of hydrocarbons and their derivatives. While Zelinsky’s primary research interests were not directly related to the Hell-Volhard-Zelinsky reaction, he made valuable contributions to the understanding of the underlying principles of organic reactions, which indirectly contributed to the development of the reaction.
The Hell-Volhard-Zelinsky reaction is a remarkable example of how scientific breakthroughs often involve the collective efforts of multiple researchers. Each of these chemists brought their unique expertise and insights to the table, leading to the development of this valuable and widely used chemical reaction.
See more new information: countrymusicstop.com
Hell Volhard Zelinsky Reaction Amino Acid | Which Carboxylic Acid Gives The Hell Volhard-Zelinsky Reaction?
The Hell-Volhard-Zelinsky (HVZ) reaction is a pretty neat chemical reaction that lets us make alpha-halo carboxylic acids. It’s a big deal in organic chemistry because it’s a key step in making lots of other important compounds, including amino acids.
Now, you might be thinking, “Why should I care about alpha-halo carboxylic acids?” Well, they’re incredibly versatile. These little guys are the building blocks for all sorts of things, including pharmaceuticals, pesticides, and even polymers.
The HVZ Reaction: A Step-by-Step Breakdown
Let’s break down how this reaction works:
1. Start with a carboxylic acid: The HVZ reaction begins with a carboxylic acid, which is basically a compound that has a carboxyl group (-COOH).
2. Add phosphorus tribromide (PBr3) or phosphorus pentachloride (PCl5): This is where things get interesting. We use either phosphorus tribromide or phosphorus pentachloride to convert the carboxylic acid into an acyl halide. This is a key step because it makes the carboxyl group more reactive.
3. Halogenation: Now, we’re ready for the main event – halogenation. We add a halogen, like bromine or chlorine, to the alpha position of the carboxylic acid. The alpha position is the carbon atom directly adjacent to the carboxyl group. This is where the “alpha-halo” part of the name comes in.
4. Hydrolysis: The final step is hydrolysis. We use water (H2O) to get rid of any leftover phosphorus halides and to convert the acyl halide back into a carboxylic acid.
Applying the HVZ Reaction to Amino Acid Synthesis
Now, let’s talk about how we can use the HVZ reaction to make amino acids.
Amino acids are the building blocks of proteins, and they have a carboxylic acid group and an amino group (-NH2). The HVZ reaction comes into play because we can use it to create an alpha-halo carboxylic acid, which can then be reacted with ammonia (NH3) to form an amino acid.
Here’s the general reaction:
Alpha-halo carboxylic acid + Ammonia → Amino acid
Specific Example: Synthesizing Alanine
Let’s take an example: We want to make alanine, a simple amino acid. We can start with propionic acid and follow these steps:
1. Convert propionic acid to an acyl halide: Using PBr3, we convert propionic acid into propionyl bromide.
2. Halogenation: We then add bromine to the alpha position, forming alpha-bromopropionic acid.
3. Reaction with ammonia: The alpha-bromopropionic acid is then reacted with ammonia, replacing the bromine atom with an amino group and giving us alanine.
Why is the HVZ Reaction So Useful?
The HVZ reaction is a powerful tool because it allows us to introduce a variety of functional groups at the alpha position of carboxylic acids. This means we can create a wide range of compounds with unique properties.
For example, we can use the HVZ reaction to synthesize:
* Alpha-amino acids: Like alanine, glycine, and valine, which are essential components of proteins.
* Alpha-halo esters: Used to make polymers and pharmaceuticals.
* Alpha-keto acids: These are involved in various metabolic pathways.
Limitations of the HVZ Reaction
While the HVZ reaction is a valuable tool, it’s not perfect. It has some limitations:
* Only works with certain carboxylic acids: The HVZ reaction only works well with carboxylic acids that have at least one alpha hydrogen atom.
* Can be difficult to control: The reaction can sometimes be difficult to control, leading to unwanted side products.
* Requires strong reagents: The reaction requires the use of strong reagents like phosphorus halides, which can be corrosive and hazardous.
The HVZ Reaction: A Powerful Tool in Organic Chemistry
Overall, the HVZ reaction is a powerful tool in organic chemistry that allows us to synthesize a wide variety of important compounds, including amino acids. It’s a crucial reaction that plays a vital role in the development of new drugs, materials, and technologies.
FAQs
1. What are some common applications of the Hell-Volhard-Zelinsky reaction in organic synthesis?
The Hell-Volhard-Zelinsky reaction is a versatile tool for creating functionalized carboxylic acids, which are valuable building blocks for many organic compounds. Some common applications include:
* Synthesis of alpha-amino acids: The HVZ reaction allows the introduction of an amino group at the alpha position of carboxylic acids, leading to the synthesis of essential amino acids like alanine, glycine, and valine.
* Synthesis of alpha-halo esters: These compounds find applications in polymer chemistry and pharmaceutical synthesis.
* Synthesis of alpha-keto acids: These are important intermediates in metabolic pathways and can be synthesized via the HVZ reaction.
2. What are the advantages and disadvantages of using the Hell-Volhard-Zelinsky reaction?
Advantages:
* Highly selective: The reaction is relatively selective for the alpha position of carboxylic acids, allowing for precise functionalization.
* Versatile: The HVZ reaction can be used to introduce a variety of functional groups at the alpha position, including halogens, amino groups, and others.
* Widely applicable: It can be applied to a range of carboxylic acids, making it a useful tool in organic synthesis.
Disadvantages:
* Limited substrate scope: The reaction only works well with carboxylic acids containing at least one alpha hydrogen atom.
* Requires strong reagents: The reaction utilizes strong reagents like phosphorus halides, which can be corrosive and hazardous to handle.
* Can be difficult to control: The reaction can sometimes be difficult to control, leading to unwanted side products.
3. What safety precautions should be taken when performing the Hell-Volhard-Zelinsky reaction?
The Hell-Volhard-Zelinsky reaction involves the use of strong reagents like phosphorus halides, which are corrosive and hazardous. Therefore, it’s crucial to take appropriate safety precautions, including:
* Working in a well-ventilated area: Phosphorus halides release corrosive fumes.
* Wearing appropriate personal protective equipment (PPE): This includes gloves, lab coat, and safety goggles to prevent contact with the reagents.
* Handling reagents with care: Use proper techniques to avoid spills and splashes.
* Disposing of waste properly: Follow appropriate procedures for disposing of hazardous chemicals.
4. What are some alternative methods for synthesizing alpha-halo carboxylic acids?
While the Hell-Volhard-Zelinsky reaction is a popular choice, alternative methods for synthesizing alpha-halo carboxylic acids exist:
* Halogenation using N-bromosuccinimide (NBS): NBS is a milder and more selective reagent for bromination.
* Addition of a halogen to an alkene: This method involves the addition of a halogen across the double bond of an alkene.
* Using a catalytic method: Certain transition metal catalysts can facilitate the selective halogenation of carboxylic acids.
The choice of method depends on the specific substrate, desired product, and the experimental conditions.
Hell-Volhard-Zelinsky reaction – Chemistry LibreTexts
The Hell Volhard Zelinsky reaction demonstrates a method for alpha addition with a carboxylic acid. The gist of the method is to Chemistry LibreTexts
Hell-Volhard-Zelinsky Reaction – Organic Chemistry
Mechanism of the Hell-Volhard-Zelinsky Reaction. Phosphorus reacts with bromine to give phosphorus tribromide, and in the first step this converts the carboxylic acid into an acyl bromide. An acyl bromide can Organic Chemistry Portal
Hell-Volhard-Zelinsky Reaction – Chemistry Learner
The Hell-Volhard-Zelinsky (HVZ) reaction is an organic reaction that is used to convert a carboxylic acid consisting of alpha hydrogen to an α-halo carboxylic acid, using a phosphorous catalyst, halogen gas, and water. Chemistry Learner
Hell-Volhard-Zelinsky (HVZ) Reaction
Hell-Volhard-Zelinsky (HVZ) Reaction. The Hell-Volhard-Zelinsky reaction effects the synthesis of α-halogenated carboxylic acids. These are useful synthetic Pomona College
Amino Acid Synthesis and Protection Reactions – OrgoSolver
One method involves using the Hell-Volhard-Zelinsky (HVZ) reaction in combination with the Gabriel synthesis, which involves the transformation of alpha-halo acids or esters OrgoSolver
Hell volhard zelinsky reaction, mechanism and examples
Hell Volhard Zelinsky is a reaction between aliphatic acids having at least one alpha-hydrogen and bromine in presence of phosphorus and phosphorus tribromide chemistnotes.com
Hell-Volhard-Zelinskii Reaction – Chemistry LibreTexts
However, carboxylic acids, can be brominated in the alpha position with a mixture of Br 2 and PBr 3 in a reaction called the Hell-Volhard-Zelinskii reaction. The Chemistry LibreTexts
Hell-Volhard-Zelinsky Reaction | Chem-Station Int. Ed.
General Characteristics. The classical method to convert carboxylic acids into α-haloacyl halides using phosphorus (III) halide is known as the Hell-Volhard-Zelinsky Chem-Station Int. Ed.
Hell-Volhard-Zelinksy Rxn And Mechanism
Understanding The Significance And Mechanism Of The Hell-Volhard-Zelinsky Reaction
Hell-Volhard-Zelinsky Reaction
21.3B The Hvz Reaction
Question-Answer (Part-1) Synthesis Of Amino Acids, Hell-Volhard-Zelinsky (Hvz Reaction) Iit Jam Net
Link to this article: hell volhard zelinsky reaction amino acid.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
