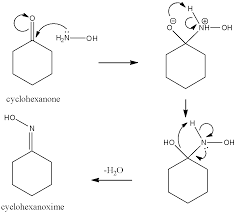
What is the mechanism of reaction of hydroxylamine?
Hydroxylamine reacts with cytosine and its derivatives in a fascinating way. This reaction occurs in a pH range of 4 to 7 and involves both first-order and second-order reactions with respect to the amine. The reaction primarily proceeds through the protonated form of the pyrimidine bases.
Let’s break this down a bit further to understand how this reaction works:
Protonation: The first step involves the protonation of the pyrimidine bases. This makes them more susceptible to attack by hydroxylamine. The protonation is driven by the acidic conditions of the reaction environment.
Nucleophilic Attack: Hydroxylamine acts as a nucleophile, meaning it has a pair of electrons that it can donate to form a new bond. It attacks the carbon at position 6 of the pyrimidine ring, which is electrophilic due to the electron-withdrawing effect of the nitrogen atoms in the ring.
Formation of the Intermediate: The attack of hydroxylamine on the carbon at position 6 results in the formation of a cyclic intermediate with a five-membered ring. This intermediate is unstable and quickly breaks down to form the final product.
Product Formation: The breakdown of the intermediate results in the replacement of the amino group at position 4 of the pyrimidine ring with a hydroxyl group. This is the key step in the reaction, leading to the formation of the modified base.
The reaction rate depends on the concentration of both hydroxylamine and the pyrimidine base. This is why the reaction is described as both first-order and second-order. The reaction rate is faster at higher concentrations of both reactants.
Understanding the mechanism of hydroxylamine reaction with cytosine and its derivatives is important for several reasons:
DNA Damage and Repair: Hydroxylamine can induce mutations in DNA by modifying cytosine. This is because the reaction product, hydroxycytosine, is prone to mispairing with adenine during DNA replication. Understanding this reaction helps us understand how DNA damage occurs and how cells repair it.
Chemical Biology: Hydroxylamine is used as a reagent in chemical biology to modify DNA and RNA. This allows researchers to study the structure and function of these molecules.
It’s important to remember that this is a simplified explanation of a complex process. There are many other factors that can influence the reaction rate and product formation. Nevertheless, understanding the fundamental steps of the reaction provides valuable insight into the chemical modifications that can occur in DNA and RNA.
What happens when formaldehyde reacts with hydroxylamine?
The most abundant amino acid produced in this reaction is glycine. Glycine is the simplest amino acid and plays a crucial role in many biological processes, such as protein synthesis and the formation of collagen.
Here’s a deeper dive into the reaction and its implications:
The reaction between formaldehyde and hydroxylamine is a complex process involving multiple steps. Initially, formaldehyde reacts with hydroxylamine to form a formaldehyde oxime intermediate. This intermediate is unstable and readily undergoes further reactions. One of the key reactions involves the condensation of two formaldehyde oxime molecules, leading to the formation of a dimeric compound. This dimeric compound can then undergo hydrolysis, yielding glycine and other byproducts.
The formation of amino acids, hydroxy acids, and other biochemical compounds in this reaction is fascinating because it provides a glimpse into how complex organic molecules might have formed in the early Earth’s environment. This reaction highlights the potential for simple organic molecules to react and form more complex structures under prebiotic conditions.
The presence of glycine as a major product is particularly noteworthy because it suggests that the building blocks for life could have been generated from simple organic molecules. This reaction provides valuable insights into the origins of life and the potential for life to arise in other parts of the universe.
What is the action of aldehyde with hydrazine?
Hydrazones are compounds that contain a nitrogen-nitrogen double bond and a carbon-nitrogen single bond. They are often used as intermediates in organic synthesis. For example, hydrazones can be further converted to the corresponding alkane by reaction with base and heat. This process is called the Wolff-Kishner reduction.
Let’s break down the reaction of aldehydes and ketones with hydrazine in more detail.
The reaction mechanism: The reaction of aldehydes and ketones with hydrazine is a nucleophilic addition reaction. The hydrazine molecule acts as a nucleophile and attacks the carbonyl carbon of the aldehyde or ketone. This results in the formation of a tetrahedral intermediate. The tetrahedral intermediate then collapses, forming the hydrazone.
The Wolff-Kishner reduction: The Wolff-Kishner reduction is a reaction that converts hydrazones to alkanes. The reaction is typically carried out in the presence of a strong base, such as potassium hydroxide (KOH). The strong base deprotonates the nitrogen atom of the hydrazone, forming an anion. This anion then undergoes a series of steps, including elimination of nitrogen gas and formation of an alkane.
Applications of the reaction: The reaction of aldehydes and ketones with hydrazine has a number of important applications in organic synthesis. For example, the reaction can be used to:
* Convert aldehydes and ketones to alkanes
* Synthesize complex organic molecules
* Study the reactivity of carbonyl compounds
Overall, the reaction of aldehydes and ketones with hydrazine is a versatile and useful reaction in organic chemistry. The reaction can be used to synthesize a variety of different compounds, including alkanes, hydrazones, and other functional groups.
How does hydroxylamine work?
This means that hydroxylamine can donate electrons to other molecules, causing them to gain electrons and be reduced. In the blood, this process leads to the formation of methemoglobin, a form of hemoglobin that cannot carry oxygen as efficiently as normal hemoglobin.
Hydroxylamine also contributes to the creation of Heinz bodies, which are abnormal structures within red blood cells that can hinder their ability to function properly.
While these effects might seem negative at first, they are actually part of a complex interplay of chemical reactions within the body. Hydroxylamine can also inhibit platelet aggregation, which is the clumping together of platelets, preventing the formation of blood clots.
Additionally, hydroxylamine acts as a nitric oxide vasodilator, meaning it helps to relax blood vessels and improve blood flow.
Let’s delve a little deeper into how hydroxylamine works.
The formation of methemoglobin and Heinz bodies: When hydroxylamine enters the bloodstream, it interacts with the iron atoms in hemoglobin, the protein responsible for carrying oxygen in red blood cells. This interaction causes the iron atoms in hemoglobin to change their oxidation state, transforming them into methemoglobin.
Methemoglobin cannot bind to oxygen as effectively as normal hemoglobin, leading to a condition known as methemoglobinemia.
Heinz bodies are aggregates of denatured hemoglobin that form when hydroxylamine interacts with the globin chains of hemoglobin. These aggregates can damage the red blood cells, leading to hemolytic anemia, a condition where red blood cells are prematurely destroyed.
Inhibition of platelet aggregation: Hydroxylamine’s ability to inhibit platelet aggregation is beneficial in preventing blood clots. Platelets are tiny cell fragments that play a crucial role in blood clotting. When a blood vessel is injured, platelets aggregate at the site of injury, forming a plug to stop bleeding.
However, in some cases, excessive platelet aggregation can lead to the formation of unwanted blood clots that can block blood vessels, leading to serious health problems.
Hydroxylamine can prevent excessive platelet aggregation by interfering with the signaling pathways that regulate platelet activation.
Nitric oxide vasodilation: Hydroxylamine’s ability to act as a nitric oxide vasodilator is another important aspect of its function. Nitric oxide is a gas that acts as a signaling molecule in the body, playing a role in various physiological processes, including blood vessel relaxation.
Hydroxylamine can enhance nitric oxide production or increase its bioavailability, leading to vasodilation and improved blood flow.
While hydroxylamine’s effects on the body are complex, understanding its role as a reducing agent, its involvement in methemoglobin and Heinz body formation, and its influence on platelet aggregation and nitric oxide vasodilation provides valuable insights into its various functions and potential applications.
What is the chemical decomposition of hydroxylamine?
You’re probably wondering what happens when hydroxylamine breaks down. Well, it’s not a simple process. It’s a complex reaction that produces a variety of gases. The overall reaction can be summarized as:
157 NH2OH(liq) → 61 NH3(gas) + 35 N2(gas) + 12 N2O(gas) + 2 NO(gas) + 143 H2O(gas) + H2(gas)
This equation basically says that when hydroxylamine decomposes, it produces ammonia (NH3), nitrogen (N2), nitrous oxide (N2O), nitric oxide (NO), water (H2O), and hydrogen (H2).
But what exactly is happening at a molecular level? It’s a bit more complicated than just one single reaction. It’s actually a bunch of smaller reactions happening at the same time, which we call a series and parallel reaction mechanism.
Think of it like a branching path in a maze. Each pathway represents a different reaction that hydroxylamine can take. And these pathways can lead to different products, like those listed in the overall equation.
To put it simply, the decomposition of hydroxylamine is a complex process that involves several reactions happening simultaneously. The final products are the result of these various reactions, and understanding the individual pathways is key to understanding the entire process.
Why does carboxylic acid not give reaction of aldehyde and ketone?
The lone pairs on the oxygen atom attached to the hydrogen atom in the -COOH group are involved in resonance, which essentially means they’re constantly shifting around. This electron movement makes the carbon atom in the carboxyl group less electrophilic, meaning it’s less likely to attract electrons and participate in reactions.
Think of it like this: the resonance creates a kind of “shield” around the carbon atom, making it less susceptible to attack. In contrast, the carbon atom in aldehydes and ketones is more open to attack due to its partial positive charge.
Let’s break it down a bit further. When a carboxylic acid reacts, it usually involves the hydroxyl group (-OH) instead of the carbonyl group. This is because the resonance stabilizes the carboxylate ion (formed when the hydroxyl group loses a proton), making the reaction more favorable.
You might wonder, why is resonance so powerful? It’s all about stability. Resonance essentially delocalizes the electrons, spreading them out over a larger area. This spreading out of electrons creates a more stable configuration, making the molecule less reactive.
In conclusion, carboxylic acids don’t behave like aldehydes and ketones because of the resonance in their carboxyl group. This resonance makes the carbon atom less electrophilic and therefore less prone to attack. This is why carboxylic acids typically react through their hydroxyl group instead of the carbonyl group, as this leads to more stable products.
See more here: What Is The Mechanism Of Reaction Of Hydroxylamine? | Hydroxylamine Reaction With Aldehyde Mechanism
What happens when aldehyde reacts with alkali?
Aldehyde reactions with alkali
Aldehydes are a class of organic compounds with the general formula RCHO, where R represents a hydrogen atom or a hydrocarbon group. They react with alkali in a variety of ways, depending on the specific aldehyde and the reaction conditions.
Cannizzaro Reaction
Aldehydes without alpha hydrogen atoms undergo a disproportionation reaction in the presence of concentrated alkali. This reaction, known as the Cannizzaro reaction, produces a mixture of an alcohol and a carboxylic acid.
Example: The Cannizzaro reaction of formaldehyde (HCHO):
In this reaction, two molecules of formaldehyde react with sodium hydroxide (NaOH) to produce one molecule of methanol (CH3OH) and one molecule of sodium formate (HCOONa).
HCHO + NaOH → CH3OH + HCOONa
Aldol Condensation
Aldehydes with alpha hydrogen atoms undergo a different type of reaction with alkali. They participate in a process called Aldol condensation, leading to the formation of beta-hydroxy aldehydes. These beta-hydroxy aldehydes are often unstable and can easily lose a molecule of water to form an unsaturated aldehyde.
Example: The Aldol condensation of acetaldehyde (CH3CHO):
Two molecules of acetaldehyde react with sodium hydroxide (NaOH) to produce a beta-hydroxy aldehyde, 3-hydroxybutanal. This beta-hydroxy aldehyde then loses a molecule of water to form an unsaturated aldehyde, but-2-enal.
CH3CHO + CH3CHO → CH3CH(OH)CH2CHO → CH3CH=CHCHO + H2O
Understanding the mechanism
The Cannizzaro and Aldol condensation reactions have unique mechanisms. The Cannizzaro reaction involves a nucleophilic attack by a hydroxide ion on the carbonyl carbon atom of the aldehyde. This leads to the formation of a tetrahedral intermediate, which then undergoes a series of steps to yield the final products.
The Aldol condensation reaction, on the other hand, involves the formation of an enolate anion, a nucleophile, which attacks the carbonyl carbon atom of another aldehyde molecule. This results in the formation of a beta-hydroxy aldehyde.
Key takeaways
Aldehydes exhibit diverse reactions with alkali, leading to various products. The type of reaction depends on the specific aldehyde and the reaction conditions.
Cannizzaro reaction:
– Occurs with aldehydes without alpha hydrogen atoms.
– Produces an alcohol and a carboxylic acid.
Aldol condensation:
– Occurs with aldehydes possessing alpha hydrogen atoms.
– Produces beta-hydroxy aldehydes, which can further dehydrate to unsaturated aldehydes.
Understanding these reactions is fundamental to comprehending the reactivity of aldehydes in organic chemistry.
See more new information: countrymusicstop.com
Hydroxylamine Reaction With Aldehyde Mechanism | What Is The Mechanism Of Aldehyde With Hydroxylamine?
Beckmann Rearrangement – Master Organic Chemistry
Mechanism: The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to formation of Master Organic Chemistry
Oxime formation – ChemTube3D
Reaction of aldehydes and ketones with hydroxylamine gives oximes. The nucleophilicity of the nitrogen on the hydroxylamine is increased by the presence of the oxygen. ChemTube3D
Formation of oximes and hydrazones (video) | Khan Academy
How aldehydes and ketones can react with hydroxylamine to form oximes or hydrazine to form hydrazones. Created by Jay. Khan Academy
The Wolff-Kishner Reaction – Chemistry LibreTexts
Aldehydes and ketones can be converted to a hydrazone derivative by reaction with hydrazine. Hydrazone formation is a variation of the imine forming reaction discussed in the previous section. Chemistry LibreTexts
18.8: The Reactions of Aldehydes and Ketones with
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is Chemistry LibreTexts
The reaction of an aldehyde with hydroxylamine gives a product
An unknown aldehyde $$’A’$$ on reacting with alkali gives a $$\beta$$- hydroxy aldehyde, which loses water to form an unsaturated aldehyde, $$2-$$ butanal. Another Toppr
Oximes – Definition, Structure, Properties along with
When an aldehyde or ketone reacts with hydroxylamine (NH 2 OH) in a weakly acidic medium, it produces oxime and eliminates water molecules. 1. Acetaldehyde reacts with BYJU’S
Oxime synthesis by condensation or oxidation – Organic
The reaction of N -alkylhydroxylamines and aqueous hydroxylamine with monosubstituted allenes gives nitrones and oximes, respectively, in good yields. DFT calculations support Organic Chemistry Portal
Oximation of aldehydes and ketones with
Oximes are important functional groups in organic chemistry due to their synthetic utility as protecting groups for carbonyl groups and their ability to form other functionalities, 1 4 and their… ResearchGate
Action Of Hydroxylamine On Aldehyde And Ketone – Aldehydes, Ketones And Carboxylic Acids
Formation Of An Oxime From An Aldehyde
Protecting Groups, Acetals, And Hemiacetals
Why Aldehyde Reacts With Nh2Oh In Acidic Medium| Aldehyde And Ketone Class 12 | With Hydroxylamine
Acetal And Hemiacetal Formation Reaction Mechanism From Aldehydes And Ketones
Link to this article: hydroxylamine reaction with aldehyde mechanism.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
