Which of the following is more basic than aniline?
Let’s break down why benzyl amine is more basic than aniline:
Electron-donating groups: The benzyl group is considered an electron-donating group because of its +I effect. This effect arises from the ability of the benzyl group to release electron density towards the nitrogen atom in the amine group. The +I effect is a result of the inductive effect, which describes the polarization of sigma bonds due to the electronegativity difference between atoms.
Electron density and basicity: The more electron density around the nitrogen atom in an amine, the more likely it is to accept a proton, making it more basic. Think of it like this: the more negatively charged the nitrogen atom is, the more it wants to attract a positively charged proton.
Aniline’s phenyl group: In contrast to the benzyl group, the phenyl group in aniline has a -I effect (electron-withdrawing effect). This effect pulls electron density away from the nitrogen atom, making it less likely to accept a proton.
In summary: The +I effect of the benzyl group in benzyl amine increases the electron density around the nitrogen atom, making it more basic compared to aniline, where the phenyl group has a -I effect, reducing the electron density around the nitrogen atom and making it less basic.
What is the solvent for Gabriel synthesis?
Dimethyl sulfoxide (DMSO), hexamethylphosphoramide (HMPA), acetonitrile, and ethylene glycol all work too. Each has its own pros and cons, and the best choice depends on the specific reaction conditions.
Here’s a breakdown of each solvent and why it might be chosen:
Dimethylformamide (DMF): DMF is a popular choice because it’s a good solvent for a wide range of compounds and it’s relatively inexpensive. It’s also a good solvent for the potassium phthalimide, which is a key reagent in the Gabriel synthesis.
Dimethyl sulfoxide (DMSO): DMSO is a stronger solvent than DMF and can dissolve a wider range of compounds. It’s often used for reactions that are difficult to perform in DMF. However, DMSO is more toxic than DMF, so it’s important to use it with caution.
Hexamethylphosphoramide (HMPA): HMPA is an even stronger solvent than DMSO. It’s often used for reactions that are very difficult to perform in DMF or DMSO. However, HMPA is very toxic, so it’s rarely used unless it’s absolutely necessary.
Acetonitrile: Acetonitrile is a good solvent for many reactions, including Gabriel synthesis. It’s less toxic than DMF or DMSO, and it’s also relatively inexpensive.
Ethylene glycol: Ethylene glycol is a polar protic solvent, which means it can dissolve both polar and non-polar compounds. It’s often used for reactions that are difficult to perform in DMF or DMSO.
Why are there so many different solvents? Because the Gabriel synthesis can be a little finicky. It involves a delicate balance of reactants and reaction conditions, so the solvent you choose can really affect how well the reaction works. The right solvent can help your reaction go faster, give you a better yield of product, or even prevent side reactions from happening.
What are the limitations of Gabriel phthalimide synthesis?
However, the reaction is not suitable for forming secondary or tertiary amines because the N-alkylphthalimide is not a good nucleophile. This means that it cannot be further alkylated. Additionally, the reaction is not suitable for forming aryl amines, because the aryl halides are not reactive enough to be attacked by the phthalimide anion.
Why can’t the Gabriel Phthalimide Synthesis make secondary or tertiary amines?
Think of it like building a chain. The Gabriel Phthalimide Synthesis gives you one link at a time (a primary amine). It’s like having a single brick that you can’t use to build anything else. To get a secondary or tertiary amine, you’d need to add more links to the chain, which is not possible with this method.
Let’s break it down further:
Secondary Amines: To make a secondary amine, you’d need to add another alkyl group to the primary amine. However, the phthalimide group makes the nitrogen less reactive, so it can’t easily react with another alkyl halide.
Tertiary Amines: This is even more challenging because you need to add two more alkyl groups. The phthalimide group makes the nitrogen *so* unreactive that it’s almost impossible to add more alkyl groups.
Imagine trying to build a house with only one brick. You can’t make a wall or a floor without more bricks. Similarly, you can’t build a secondary or tertiary amine with the Gabriel phthalimide synthesis because it only gives you one “brick” (a primary amine).
What is Robinson Gabriel synthesis used for?
The key is that you need to start with certain compounds, and then add either acid or POCl3, which acts like a catalyst to help the reaction happen. The result is a new molecule called an oxazole, which has a unique structure that makes it useful for various applications.
Oxazoles are important building blocks in organic chemistry, and they have many uses, from making pharmaceuticals to developing new materials. For example, some oxazoles are used in antibiotics and antifungal drugs. This is because they have biological activity, meaning they can interact with living systems, which makes them useful for treating infections.
You can also find oxazoles in agrochemicals, which are substances that help protect crops from pests and diseases. They are even used to create dyes and pigments, adding vibrant colors to textiles and paints.
See more here: What Is Gabriel Phthalimide Synthesis Only For? | Gabriel Synthesis Is Used For The Preparation Of
See more new information: countrymusicstop.com
Gabriel Synthesis Is Used For The Preparation Of | Which Of The Following Can Be Prepared By Gabriel Synthesis?
Gabriel Synthesis – Chemistry LibreTexts
The goal of Gabriel synthesis is to create a primary amine (RNH 2). Doing a direct S N 2 reaction would result in a quaternary amine salt, which can be pretty useless (unless you use Hofmann elimination). Chemistry LibreTexts
The Gabriel Synthesis – Master Organic Chemistry
The Gabriel synthesis of amines is the reaction of a phthalimide salt with an alkyl halide followed by hydrolysis. Examples, mechanism, and more below. Skip to content Master Organic Chemistry
Gabriel Phthalimide Synthesis Mechanism – Explanation
What is Gabriel Phthalimide Synthesis Reaction? Gabriel Phthalimide Synthesis Mechanism has 3 steps. The Synthesis is used to get primary amines from primary alkyl halides and is named after the German BYJU’S
The Gabriel Synthesis: A Method for Producing Primary Amines
The Gabriel Synthesis is an essential organic chemistry technique developed by Siegmund Gabriel for synthesizing primary amines from alkyl halides. It utilizes algoreducation.com
Gabriel Synthesis – an overview | ScienceDirect Topics
Gabriel Synthesis. The Gabriel synthesis is used to convert a haloalkane into an amine in which the amino group replaces the halogen. From: Organic Chemistry Study Guide, 2015 ScienceDirect
Gabriel Synthesis | Overview & Research Examples – Perlego
Gabriel Synthesis is a method used to prepare primary amines from potassium phthalimide and alkyl halides. The reaction involves the formation of an intermediate, Perlego
Gabriel Synthesis – Organic Chemistry Portal
Gabriel Synthesis. Potassium phthalimide is a -NH 2-synthon which allows the preparation of primary amines by reaction with alkyl halides. After alkylation, the phthalimid is not nucleophile and does not react anymore. Organic Chemistry Portal
The Gabriel Synthesis – Chemistry Steps
Let’s put together a summary of the Gabriel reaction for preparing1° amines in which the overall result is nucleophilic substitution of X by NH 2, so the Gabriel synthesis can be used: Now, the amide hydrolysis is often Chemistry Steps
Gabriel Synthesis | Thermo Fisher Scientific – US
Learn about the Gabriel synthesis nucleophilic substitution reaction that involves a two-step process, alkylation followed by hydrolysis, to prepare primary amines from their Thermo Fisher Scientific
Gabriel Synthesis Reaction Mechanism – Alkyl Halide To Primary Amine
Gabriel Amine Synthesis
Gabriel Synthesis Is Used For The Preparation Of
Gabriel Phthalimide Synthesis Is Used For The Preparation Of (A) Primary Aromatic Amines (B) Pri…
Gabriel Synthesis Mechanism For Primary Amines
Link to this article: gabriel synthesis is used for the preparation of.
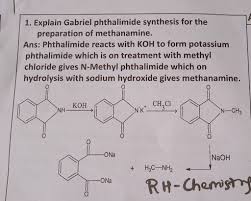
See more articles in the same category here: blog https://countrymusicstop.com/wiki
