Which is the major product of ortho-nitrophenol or para nitrophenol?
Ortho-nitrophenol is more stable than para-nitrophenol due to a special interaction called intramolecular hydrogen bonding. This means that within the same molecule, the hydrogen atom of the OH group forms a bond with one of the oxygen atoms in the nitro group. This bond isn’t as strong as a regular chemical bond, but it creates a kind of “hug” that stabilizes the molecule.
Para-nitrophenol, on the other hand, doesn’t have this “hug” because the nitro group is too far away from the OH group. Think of it like this: ortho-nitrophenol is like a pair of friends holding hands, while para-nitrophenol is like friends standing across the street from each other. The friends holding hands are more stable, just like ortho-nitrophenol.
Now, let’s talk about why this stability matters when making nitrophenols. The reaction to make nitrophenols involves a nitration step, where a nitro group is added to a phenol molecule. This reaction can happen at different positions on the phenol ring, and it turns out that ortho-nitrophenol is the preferred product because it’s more stable.
You might be wondering, “If ortho-nitrophenol is so stable, why do we even get any para-nitrophenol?” That’s a great question! The answer lies in the fact that nitration is a complex process that involves many factors. One of these factors is steric hindrance. This means that the large nitro group can sometimes get in the way of adding a nitro group to the ortho position, making it easier to add to the para position instead.
In summary, while para-nitrophenol can be formed, ortho-nitrophenol is the major product because it’s more stable due to intramolecular hydrogen bonding. This extra stability makes it more favorable for the reaction to produce ortho-nitrophenol.
What is the major product when phenol is reacted with dilute hno3?
Let’s dive deeper into the reasons behind this preference for the ortho isomer.
The reaction of phenol with HNO3 is an electrophilic aromatic substitution reaction. In this reaction, the nitronium ion (NO2+) acts as the electrophile, attacking the aromatic ring of phenol. Now, the OH group on the phenol ring is an electron-donating group. This means it increases the electron density in the aromatic ring, making it more susceptible to attack by electrophiles.
However, the OH group also directs the incoming electrophile to the ortho and para positions. This is due to the resonance structures that can be drawn for phenol. These resonance structures show that the OH group donates electron density to the ortho and para positions. This electron density makes these positions more reactive towards electrophilic attack.
But, why is ortho-nitrophenol the major product? This is because the ortho position is sterically less hindered than the para position. The bulky NO2 group experiences less steric hindrance when it occupies the ortho position.
Therefore, despite the para position also being activated, the ortho position wins out due to the lesser steric hindrance, making ortho-nitrophenol the major product.
What are the major products in the nitration of phenol?
Let’s break down why this happens. The reaction is an electrophilic aromatic substitution. The nitric acid acts as the electrophile, attacking the aromatic ring of phenol. Now, phenol is a very reactive molecule, and the hydroxyl group attached to the ring directs the incoming electrophile to the ortho and para positions.
Why does ortho-nitrophenol form in higher amounts? The answer lies in the stability of the intermediate formed during the reaction. The ortho position offers a more stable intermediate due to the hydrogen bonding between the hydroxyl group on the phenol and the nitro group on the newly formed ortho-nitrophenol. This stabilization is not possible for the para isomer. Therefore, the formation of ortho-nitrophenol is favored, leading to its higher yield.
Here’s a simple way to visualize it:
ortho-nitrophenol: The nitro group is adjacent to the hydroxyl group, allowing for hydrogen bonding and greater stability.
para-nitrophenol: The nitro group is across from the hydroxyl group, so there’s no direct hydrogen bonding.
The difference in stability is subtle, but it has a big impact on the final product distribution!
What is the major product of phenol?
You see, phenol reacts with sodium hydroxide to form sodium phenoxide. This phenoxide ion is more reactive than phenol, making it a prime target for electrophilic substitution. When you introduce carbon dioxide (a weak electrophile) to the mix, it reacts with the phenoxide ion to produce salicylic acid – our star product.
Now, why is salicylic acid so important? It’s a crucial ingredient in many medications, notably aspirin, a popular pain reliever. You can even find salicylic acid in acne treatments, as it has antibacterial and anti-inflammatory properties. So, the next time you use an aspirin or a topical acne treatment, remember that phenol played a role in creating the active ingredient!
Salicylic acid is an interesting compound, and it’s worth exploring further. It’s made through a process called the Kolbe-Schmitt reaction. This reaction involves treating sodium phenoxide with carbon dioxide under high pressure and temperature. The carbon dioxide molecule reacts with the phenoxide ion to form a salicylic acid molecule.
The Kolbe-Schmitt reaction is a significant example of how phenol can be used to create valuable compounds. It’s a testament to the versatility of phenol in the world of chemistry.
What is the major product of nitration of chlorobenzene?
You might be wondering why these two, right? Well, the nitro group is an electron-withdrawing group, which means it pulls electrons away from the benzene ring. This makes the ring less reactive towards electrophilic aromatic substitution, but it also directs the incoming nitro group to specific positions.
Chlorine is also an electron-withdrawing group, but it’s weaker than the nitro group. This means that it will slightly deactivate the ring but also direct the incoming group to the ortho and para positions.
So, why do you get 1-chloro-2-nitrobenzene and 1-chloro-4-nitrobenzene? It’s all about the electron density in the ring. The nitro group prefers to attach to positions where the electron density is highest. The ortho and para positions have a higher electron density than the meta position, so you get those two products.
Think of it like this: chlorine is like a traffic cone, guiding the nitro group to the ortho and para lanes.
It’s important to note that the meta product, 1-chloro-3-nitrobenzene, is also formed, but in much smaller amounts. This is because the meta position is less electron-rich.
Here’s a table to help you visualize this:
| Position | Electron Density | Product |
|—|—|—|
| Ortho | High | 1-chloro-2-nitrobenzene |
| Meta | Low | 1-chloro-3-nitrobenzene |
| Para | High | 1-chloro-4-nitrobenzene |
The ortho and para products are more stable than the meta product due to the resonance stabilization of the nitro group with the benzene ring.
You can see how the nitro group can move around the ring through resonance, which contributes to its stability.
1-chloro-4-nitrobenzene is the major product because it has the nitro group in the para position, which is the most stable position. This is because it has the most resonance structures, making it the most stable.
Let me know if you have any other questions!
Which is stronger o-nitrophenol or p-nitrophenol?
o-nitrophenol forms an intramolecular hydrogen bond, essentially a bond within the same molecule. This bond involves the hydrogen atom of the hydroxyl group (OH) and one of the oxygen atoms of the nitro group (NO2). This bond makes the hydrogen less readily available for donation, weakening the acidity of o-nitrophenol.
On the other hand, p-nitrophenol doesn’t form an intramolecular hydrogen bond. The nitro group and hydroxyl group are positioned farther apart, so the hydrogen is more easily released as a proton (H+). This means p-nitrophenol readily donates a proton, making it a stronger acid.
Think of it this way: In o-nitrophenol, the hydrogen is busy forming a cozy bond within the molecule, making it harder to break free and donate a proton. In p-nitrophenol, the hydrogen is more “lonely” and readily available to donate its proton.
Here’s a breakdown of why the intramolecular hydrogen bond affects acidity:
Stabilization: The intramolecular hydrogen bond in o-nitrophenol stabilizes the molecule, making it less likely to lose a proton and become anionic.
Resonance: The nitro group in p-nitrophenol can better stabilize the negative charge that forms when the proton is donated. This stabilization makes the proton donation more favorable, increasing acidity.
In a nutshell, the key to understanding the acidity difference between o-nitrophenol and p-nitrophenol lies in the presence or absence of intramolecular hydrogen bonding and the subsequent influence on the hydrogen atom’s ability to donate a proton.
See more here: What Is The Major Product Of Phenol With Nitrous Acid? | Nitration Of Phenol Major Product
How do you nitrate phenols?
Nitrating Phenols
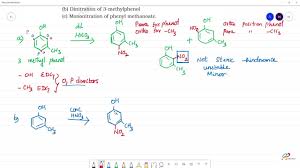
Phenols are pretty reactive, and when you treat them with dilute nitric acid at a low temperature (around 298 Kelvin), they’ll get nitrated. This means a nitro group (-NO2) will be added to the phenol molecule. The nitration reaction will produce a mixture of ortho and para nitrophenols. These are isomers, meaning they have the same chemical formula but different arrangements of atoms.
Here’s the breakdown:
Ortho Nitrophenol: The nitro group is attached to the carbon atom next to the hydroxyl group (-OH).
Para Nitrophenol: The nitro group is attached to the carbon atom opposite the hydroxyl group.
You can separate these two isomers using a technique called steam distillation. The reason this works is that ortho nitrophenol is more volatile than para nitrophenol, meaning it evaporates more easily.
Let’s dive into the details a little more:
Think of the phenol molecule as having a ring of six carbon atoms with a hydroxyl group (-OH) attached to one of them. When you add nitric acid, the nitro group is attracted to the ring. But why does it go to the ortho and para positions?
It has to do with the electron density in the ring. The hydroxyl group is electron-donating, meaning it pushes electrons towards the ring. This makes the ring more reactive, especially at the ortho and para positions. The meta position (the one directly across from the hydroxyl group) is less reactive because the electron density is lower there.
Now, why is ortho nitrophenol more volatile than para nitrophenol?
It’s a bit more complicated, but it has to do with the ability of the hydroxyl group to form hydrogen bonds. In ortho nitrophenol, the hydroxyl group can form an intramolecular hydrogen bond with the nitro group (a bond within the same molecule). This makes it harder for the molecule to escape into the gas phase, so it’s more volatile.
In para nitrophenol, the hydroxyl group can only form intermolecular hydrogen bonds (bonds between different molecules), so it’s less volatile.
The separation by steam distillation works because when you heat up the mixture, the ortho nitrophenol will evaporate more readily and can be collected in the distillate (the condensed vapor). The para nitrophenol will stay behind in the reaction vessel.
See more new information: countrymusicstop.com
Nitration Of Phenol Major Product | What Is The Major Product Of Nitro Phenol?
why is o nitrophenol a major product in nitration of phenol
Q. Phenol reacts with sodium hydroxide to give sodium phenoxide. Phenoxide ion undergoes electrophilic substitution with carbon dioxide ( a weak electrophile) because phenoxide ion is more reactive than phenol. Salicylic acid is formed as a major product. BYJU’S
7.4.5 Nitration & Bromination of Phenol – Save My Exams
Nitration is an example of an electrophilic substitution reaction; The nitration of benzene requires a mixture of concentrated nitric acid (HNO 3) and sulfuric acid (H 2 SO 4) savemyexams.com
Why is the para product major in the nitrosation of
When phenol reacts with nitrous acid ($\ce{NaNO2 + \mathrm{conc}\ H2SO4}$), 4-nitrosophenol and not 2-nitrosophenol is formed. I cannot understand why the para -isomer should be preferred Chemistry Stack Exchange
Nitration of Phenol (A-Level Chemistry) – YouTube
Outlining the nitration of phenol reaction. Differences between the nitration of benzene and the nitration of phenol are shown and explained.Recap: 00:25Nitr… YouTube
Electrophilic Substitution Reactions of Phenols
Nitration of Phenols. Phenols upon treatment with dilute nitric acid undergo nitration at low temperature (298 K) to give a mixture of ortho and para nitrophenols. The mixture formed is further separated into ortho BYJU’S
The mechanisms of nitration of phenol – ScienceDirect
Nitrosation is an electrophilic aromatic substitution involving the nitrosonium ion and is mainly para selective (refs. 4, 5), and the oxidation is due to the nitrogen ScienceDirect
Nitration of Phenols (video) | Khan Academy
About. In this video, we will learn how to prepare p-nitrophenol, a very important intermediate in the synthesis of the drug, paracetamol. Khan Academy
organic chemistry – What will be the major product in the nitration …
Generally electrophilic aromatic substitution of phenol gives para product in slightly higher amount than ortho product due to inductive effect of oxygen. However in Chemistry Stack Exchange
The mechanisms of nitration of phenol – ScienceDirect
Nitration of phenol is an old reaction that was described for the first time in 1875. Nitrophenols are of great interest for the industry because they can be used as ScienceDirect
15.2: Regioselectivity in Electrophilic Aromatic
Let’s take the nitration of phenol. One two of a possible three monosubstituted products form (ortho and para). Why not meta? Well, let’s look at the mechanism: a) ortho-nitration. b) meta-nitration. c) para Chemistry LibreTexts
What Is The Major Product In The Nitration Of Phenol
Nitration Of Phenol || #Phenol #Nitration #Jee #Neet
Predict The Major Products Formed From The Following Reactions : A) Dinitration Of 3-Methylpheno…
What Is The Major Product Of The Following Reactions?
Write The Structures Of The Major Products Expected From The Following Reactions: (A) Mononitrat…
Link to this article: nitration of phenol major product.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
