Is cis-trans isomerism possible in alkynes?
Alkynes, with their triple bonds, have a linear geometry. This means the two carbon atoms involved in the triple bond, along with the atoms directly attached to them, all lie in a straight line. Think of it like a straight road with no turns! There simply aren’t different sides or orientations for the substituents on the carbons to occupy, like you’d see in alkenes.
Imagine you have a triple bond between two carbon atoms, let’s call them C1 and C2. Each carbon atom forms one sigma bond with another atom. The remaining two bonds are the pi bonds that make up the triple bond. Since the triple bond is linear, the two pi bonds are oriented perpendicular to each other. Because of this arrangement, there’s no possibility of having different spatial arrangements of the substituents on the carbons.
For cis-trans isomerism to occur, we need a situation where the substituents on a carbon atom can be on the same side (cis) or opposite sides (trans) of a double bond. Alkynes just don’t have that kind of structure! They have a triple bond, which is linear, and there’s no way to have a cis or trans configuration.
Why do alkanes and alkynes not show cis-trans isomerism?
The key lies in the types of bonds present. Alkanes have only single bonds, which are formed by the overlap of sp3 hybridized orbitals. This allows for free rotation around the bond axis. Imagine a spinning top; the groups attached to the carbon atoms can rotate freely without breaking the bond.
Alkynes, on the other hand, have triple bonds, formed by the overlap of sp hybridized orbitals. These bonds are very strong and prevent any rotation. Picture a rigid rod; the groups attached to the carbon atoms are locked in place and cannot rotate.
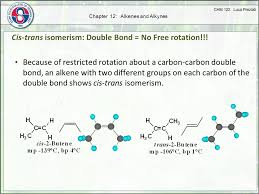
Cis-trans isomerism, also known as geometric isomerism, arises when there is restricted rotation around a bond. It’s like having two different configurations, like a pair of shoes with the left and right foot. Because alkanes have free rotation and alkynes have rigid, non-rotating triple bonds, they don’t meet the criteria for cis-trans isomerism.
To illustrate this, consider ethane (C2H6), the simplest alkane. The two methyl groups (CH3) attached to the carbon atoms can rotate freely, meaning there’s no fixed spatial arrangement. Now, take ethyne (C2H2), the simplest alkyne. The two hydrogen atoms attached to the carbon atoms are fixed in a linear arrangement due to the triple bond, preventing any rotational freedom.
Therefore, alkanes and alkynes lack the necessary structural feature (restricted rotation) for cis-trans isomerism.
Which isomers can show cis-trans isomerism?
To understand this, we need to think about what cis-trans isomerism means. It occurs when a molecule has two different groups attached to each carbon atom of a double bond. Imagine the double bond as a rigid bar, and the groups attached to it as flags. If the flags are on the same side of the bar, we call it cis. If they are on opposite sides, it’s trans.
Now, look at the structure of 2-chlorobut-2-ene. It has a chlorine atom and a methyl group (CH3) attached to each carbon of the double bond. This means there are two different groups on each side, making cis-trans isomerism possible.
However, the other isomers in this group don’t meet this condition. They might have two of the same group attached to one of the double-bonded carbons. For example, imagine one carbon has two chlorine atoms and the other has a methyl group and a hydrogen. In this case, you can’t have a cis or trans arrangement because both chlorine atoms are identical.
Here’s a way to visualize it:
Cis-2-chlorobut-2-ene: The chlorine and methyl group are on the same side of the double bond.
Trans-2-chlorobut-2-ene: The chlorine and methyl group are on opposite sides of the double bond.
The other isomers, lacking this distinct difference in groups on each carbon of the double bond, can’t form cis-trans isomers. This is why 2-chlorobut-2-ene stands out as the only isomer in this group capable of exhibiting this type of isomerism.
Is cis-trans isomerism possible in alkenes?
If the groups are on the same side of the double bond, it’s a cis isomer. Imagine two friends walking side-by-side! But if the groups are on opposite sides of the double bond, it’s a trans isomer. Think of two friends walking across from each other.
Let’s break down a simple example with the alkene R-CH=CH-R:
Cis isomer: The two R groups are on the same side of the double bond.
Trans isomer: The two R groups are on opposite sides of the double bond.
Think of it like a seesaw. If both kids are on the same side, it’s cis. If they’re on opposite sides, it’s trans!
Here’s a visual representation of what this looks like:
Cis Isomer:
“`
R
|
H—C=C—H
|
R
“`
Trans Isomer:
“`
R
|
H—C=C—H
|
R
“`
These different arrangements of the R groups give the isomers unique properties. They might have different melting points, boiling points, and even different reactivities.
It’s important to remember that cis-trans isomerism isn’t always possible in alkenes. If one of the carbons attached to the double bond has two identical groups, then there’s no difference in how the groups are arranged, and no cis-trans isomers can form.
Do triple bonds have cis-trans isomers?
Let’s delve a little deeper into why triple bonds don’t exhibit cis-trans isomerism. Imagine a molecule with a triple bond between two carbons. Each carbon atom participating in the triple bond has two remaining sp hybridized orbitals. These orbitals point in opposite directions, forming a 180-degree angle. The atoms attached to these carbons are thus located directly opposite each other, eliminating any possibility of different arrangements that would lead to cis and trans configurations.
To illustrate this further, consider the simplest example: acetylene (C2H2). In acetylene, each carbon atom is bound to one hydrogen atom. These hydrogen atoms are positioned at opposite ends of the molecule due to the linear geometry created by the triple bond. This means there’s only one possible arrangement of the atoms, and hence, there are no cis-trans isomers.
In contrast, double bonds, which have a sp2 hybridization, allow for a planar geometry, leading to the possibility of cis-trans isomers. This is because the two remaining sp2 orbitals on each carbon atom in a double bond are oriented in the same plane, allowing for different spatial arrangements of the substituents around the double bond. For instance, in dichloroethylene (C2H2Cl2), the two chlorine atoms can be on the same side of the double bond (cis isomer) or on opposite sides (trans isomer). However, this doesn’t occur with triple bonds, where the linear geometry and the arrangement of the atoms eliminates the possibility of different spatial orientations.
Is halogenation of alkynes cis or trans?
When you add the first halogen molecule to an alkyne, the major product you get is a trans-dihaloalkene. Think of it as the two halogen atoms attaching to the alkyne from opposite sides, resulting in that trans configuration. You also get a smaller amount of the cis isomer, where the halogens are on the same side.
But why is the trans product the major one? It comes down to the way the reaction happens. The addition of halogens to alkynes happens in a two-step process. First, the halogen molecule interacts with the pi bonds of the alkyne, creating what’s called a halonium ion. This ion is unstable and quickly reacts with another halide ion. The trans configuration is favored because it minimizes steric hindrance, meaning the bulky halogen atoms are less likely to bump into each other.
Adding the second equivalent of halogen to the trans-dihaloalkene gives you the tetrahalide, where you have four halogen atoms attached. So, while you might get a small amount of the cis isomer in the first step, the subsequent addition of halogen leads to a tetrahalide with all the halogens on the same side.
Remember, the addition of halogens to alkynes is a pretty important reaction in organic chemistry. It’s a great way to introduce new functional groups and create new molecules with different properties.
Which compound does not show cis-trans isomerism?
Cis-trans isomerism happens when there’s restricted rotation around a double bond. This restriction means that the groups attached to the double bond can be arranged in different ways, leading to distinct isomers. Think of it like two people on a seesaw; they can be on the same side (cis) or opposite sides (trans) of the pivot point.
Now, let’s look at 1-hexene. It’s a six-carbon chain with a double bond between the first and second carbon atoms. Here’s the key: The double bond is at the end of the chain.
Imagine a seesaw where the pivot point is at the very edge! You can’t have two people on opposite sides of the pivot because there’s no space on the other side. Similarly, in 1-hexene, since the double bond is at the end, there’s no possibility of having different spatial arrangements of the groups attached to it.
So, there’s only one possible structure for 1-hexene, and it doesn’t exhibit cis-trans isomerism. This is in contrast to molecules like 2-butene, where the double bond is in the middle of the chain, allowing for both cis and trans isomers.
Here’s a simple analogy to understand this: Think of a door hinge. If the hinge is at the end of the door, the door can only swing open in one direction. But if the hinge is in the middle of the door, it can swing open in both directions.
See more here: Why Do Alkanes And Alkynes Not Show Cis-Trans Isomerism? | Alkynes Can Show Cis Trans Isomerism
See more new information: countrymusicstop.com
Alkynes Can Show Cis Trans Isomerism: A Surprising Twist
So, the short answer is no, alkynes generally don’t exhibit cis-trans isomerism. But, hold on! There’s a catch.
Let’s break it down.
What’s Cis-Trans Isomerism, Anyway?
Cis-trans isomerism, also known as geometric isomerism, happens when you’ve got two different groups attached to each carbon of a double bond. It’s like having two different things stuck on a seesaw, and depending on how they’re oriented, you can have either a cis or trans arrangement.
Cis means the two groups are on the same side of the double bond. Think of them as buddies, sitting side-by-side.
Trans means the two groups are on opposite sides of the double bond. Like two people who really don’t get along, sitting as far away from each other as possible.
Alkynes and Their Triple Bonds
Alkynes are hydrocarbons that have a triple bond between two carbon atoms. This triple bond is made up of one sigma bond and two pi bonds.
Now, the key here is that a triple bond is linear. It’s like a straight line. And because of that straight line, there’s no possibility of having groups on the same side or opposite sides of the bond.
Imagine it like this: Think about a seesaw, but instead of a flat platform, it’s a straight line. You can’t put two people on the seesaw so they’re facing each other (cis) or facing away from each other (trans) because there’s no “side” in a straight line. It’s all just one direction.
That’s why alkynes don’t usually show cis-trans isomerism.
The Catch: Cyclic Alkynes
But remember, I said there was a catch!
There are some exceptions to this rule. Cyclic alkynes can exhibit cis-trans isomerism.
Cyclic alkynes are like regular alkynes, but they’re part of a ring structure. And because they’re in a ring, the triple bond isn’t necessarily linear. It can be bent or twisted, giving you the possibility of having groups on “different sides” of the bond.
Let me give you an example: Imagine you have a ring with a triple bond, and you attach two different groups to the carbons that make up that triple bond. You could have those groups on the same side of the ring (cis) or on opposite sides of the ring (trans).
So, in this case, you can have cis-trans isomerism.
Let’s Recap
Here are the key takeaways:
Alkynes generally don’t show cis-trans isomerism. This is because the triple bond is linear, and there’s no possibility of having groups on the same or opposite sides of the bond.
Cyclic alkynes can show cis-trans isomerism. This is because the triple bond can be bent or twisted within the ring structure, allowing for different spatial arrangements of groups.
FAQs
Q: Why are alkynes linear?
A: The triple bond in alkynes is made up of one sigma bond and two pi bonds. The sigma bond is strong and keeps the atoms in a straight line. The pi bonds, which are weaker, help to create the triple bond, but they don’t affect the linearity of the molecule.
Q: Can alkynes exhibit E/Z isomerism?
A:No, alkynes can’t exhibit E/Z isomerism. E/Z isomerism is a more general type of geometric isomerism that applies to any double bond with different groups attached to the carbons. However, because alkynes have a triple bond, there’s no “side” for the groups to be on, and therefore no E/Z isomers.
Q: What about alkenes? Do they show cis-trans isomerism?
A: Yes, alkenes do show cis-trans isomerism. Alkenes have a double bond, which is planar (flat). This allows for the possibility of having groups on the same side or opposite sides of the bond, leading to cis and trans isomers.
Q: Are there any other types of isomerism besides cis-trans isomerism?
A: Absolutely! There are a bunch of different types of isomerism, including:
Structural isomerism: This happens when molecules have the same molecular formula but different structures.
Enantiomerism: This happens when molecules are mirror images of each other and cannot be superimposed.
Diastereomerism: This happens when molecules are stereoisomers that are not enantiomers.
There’s a whole world of isomerism out there!
Cis–trans isomerism (video) | Khan Academy
Cis–trans notation can be used to describe the configuration of a double bond with exactly two substituents and two hydrogens. If the two substituents are on the same side of the Khan Academy
Cis–trans isomerism | Alkenes and Alkynes | Organic chemistry
Start practicing—and saving your progress—now! https://www.khanacademy.org/science/o… Cis–trans notation can be used to describe the configuration of a double bond with exactly two … YouTube
13.2: Cis-Trans Isomers (Geometric Isomers) – Chemistry LibreTexts
Recognize that alkenes that can exist as cis-trans isomers. Classify isomers as cis or trans. Draw structures for cis-trans isomers given their names. Chemistry LibreTexts
7.5: Cis-Trans Isomerism in Alkenes – Chemistry
In one, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. (trans: from latin meaning “across” – as in transatlantic). In the other, the two chlorine atoms are Chemistry LibreTexts
9.1: Naming Alkynes – Chemistry LibreTexts
Alkynes cannot exhibit E,Z (cis‑trans) isomerism; hence, in this sense, their nomenclature is simpler than that of alkenes. Alkynes are organic molecules made of the functional group carbon Chemistry LibreTexts
7.4 Cis–Trans Isomerism in Alkenes – Organic Chemistry
The cis isomer has the two methyl groups on the same side of the double bond, and the trans isomer has methyl groups on opposite sides. Cis–trans isomerism is not limited OpenStax
CHAPTER FOUR Alkenes and Alkynes – chemistry.sdsu.edu
Cis‐TransIsomerism – Vitamin A has five C‐C double bonds, four of which can show cis‐trans isomerism. – Vitamin A is the all‐trans isomer. Physical Properties • Alkenes sdsu.edu
Chapter 5: Alkenes and Alkynes – Michigan State
These differences make it possible to separate E and Z isomers (and cis/trans since they are just a special case of E/Z) from one another. Stability of alkenes: Elimination reactions that produce alkenes tend to Open Textbook Publishing
Difference Between Cis and Trans Isomers
Usually, cis and trans isomerism can be found in organic and inorganic compounds, alkanes and in alkenes. BYJU’S
Cis–Trans Isomerism | Alkenes And Alkynes | Organic Chemistry | Khan Academy
Cis And Trans Isomers
13A: Classifying Alkenes As Cis Or Trans
Using Cis/Trans Versus E/Z To Describe Double Bonds
4.3: Isomerism (Cis-Trans Isomer)
Link to this article: alkynes can show cis trans isomerism.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
