What is the notation for manganese?
Let’s dive a little deeper into the notation for manganese. Mn is a unique identifier that helps scientists and others recognize this element. It’s like a special code for manganese. This symbol, Mn, is used in the periodic table, which is a chart that organizes all the known elements. You’ll always see Mn representing manganese, no matter what.
Think of it like this: imagine you’re trying to write down the name of your favorite food. It’s much easier to write “pizza” instead of writing out the whole description of what it is, right? The symbol Mn is like a shortcut for manganese. It helps us talk about this element without having to write out its whole name every time.
What is magnesium noble gas notation?
Let’s break this down. Neon has an electron configuration of 1s²2s²2p⁶. The noble gas configuration of magnesium tells us that magnesium has the same inner electron configuration as neon, which is 1s²2s²2p⁶, followed by two additional electrons in the 3s orbital.
So, the full electron configuration of magnesium is 1s²2s²2p⁶3s².
The noble gas configuration is a shortcut way of writing the electron configuration of an element. It simplifies the way we represent the electrons in an atom and helps us understand the reactivity of elements. For example, magnesium has two valence electrons in its outer shell (3s orbital). These valence electrons are responsible for the chemical properties of magnesium and its tendency to lose those two electrons to form ionic bonds.
Let’s discuss how the noble gas configuration helps us understand this. Noble gases are known for their stability and unreactivity because they have a full outer shell of electrons. Magnesium, by losing its two valence electrons, can achieve the same stable electron configuration as neon. This is why magnesium tends to form +2 cations.
This ability to achieve a stable electron configuration is a core concept in chemistry and helps us predict how elements will behave in chemical reactions.
What is the noble gas notation for PB?
We can write the noble gas configuration for lead as [Xe] 6s² 4f¹⁴ 5d¹⁰ 6p². The [Xe] represents the electron configuration of xenon, and the rest of the notation tells us the arrangement of the remaining 28 electrons.
* 6s² means that there are two electrons in the 6s orbital.
* 4f¹⁴ means that there are 14 electrons in the 4f orbital.
* 5d¹⁰ means that there are 10 electrons in the 5d orbital.
* 6p² means that there are two electrons in the 6p orbital.
This noble gas configuration helps us understand the electronic structure of lead and how it interacts with other elements. For example, the two electrons in the 6p orbital are the valence electrons, which are the electrons involved in chemical bonding. This configuration explains why lead has a tendency to lose two electrons to form a +2 ion or to share its electrons to form covalent bonds.
How do you write noble gas notation?
It’s actually quite simple! You take the elemental symbol of the last noble gas before the element you’re looking at and follow it with the configuration of the remaining electrons.
For example, let’s take sodium. Its electron configuration is 1s²2s²2p⁶3s¹. We can replace the 1s²2s²2p⁶ part with the symbol of neon (Ne), the noble gas preceding sodium.
This gives us the noble gas notation of sodium: [Ne]3s¹.
How Does it Work?
Noble gases, like helium, neon, argon, krypton, xenon, and radon, are special. They have a full outer shell of electrons, making them very stable and unreactive.
When we write noble gas notation, we’re essentially recognizing this stability. We’re saying that the core electrons (the ones that are already filled up to the last noble gas) are like a stable “foundation.” The remaining electrons are the ones that really determine the element’s reactivity.
Think of it like this:
Imagine you’re building a house. You start with a solid foundation (the noble gas core) and then add the rest of the walls and rooms (the remaining electrons). The foundation is already stable, and it’s the added structure that makes the house unique.
By focusing on the “extra” electrons, we can more easily understand the element’s behavior and predict how it might bond with other elements.
Let’s try another example:
Chlorine has the electron configuration 1s²2s²2p⁶3s²3p⁵. We can replace the 1s²2s²2p⁶ part with the symbol of argon (Ar), giving us the noble gas notation of chlorine: [Ar]3s²3p⁵.
See, it’s not that hard!
What is manganese noble gas shorthand?
You’re right, manganese has an electron configuration of 1s22s22p63s23p64s23d5. This tells us how many electrons are in each energy level.
Now, here’s the cool part: instead of writing out the whole configuration, we can use noble gas shorthand. It’s like a shortcut! We find the noble gas element that comes before manganese on the periodic table, which is argon (Ar). Argon has a complete outer shell of electrons, represented by [Ar].
Since manganese has the same inner electron configuration as argon, we can simply write [Ar]4s23d5 to represent the full electron configuration.
This noble gas shorthand makes it much easier to understand where the valence electrons are located. In this case, we see that manganese has five valence electrons in the 3d subshell. This is why manganese is a transition metal with interesting chemical properties!
Think of it this way: instead of writing out the entire address of a house, we can simply say, “It’s on the same street as the post office.” The noble gas shorthand is like a postal code, providing a convenient and concise way to represent the electron configuration of an element.
In addition to the noble gas shorthand for manganese, we can also talk about the unpaired electrons. Manganese has five unpaired electrons in its 3d orbitals, which explains why it’s paramagnetic.
Paramagnetism is a property of substances that are attracted to magnetic fields. This is because the unpaired electrons in the 3d orbitals of manganese act like tiny magnets, aligning themselves with an external magnetic field.
It’s a bit like having five little magnets on a string, each spinning independently. When you apply a magnetic field, these magnets line up, making the whole string more magnetic.
So, the noble gas shorthand not only simplifies the writing of the electron configuration, but it also helps us understand the unique properties of manganese like its paramagnetism.
Which noble gas is MG?
Magnesium (Mg), with atomic number 12, is not a noble gas. It’s a metal! To achieve a stable electronic configuration, it loses two electrons to become a cation with a +2 charge. This means it ends up with the same electronic configuration as Neon (Ne), a noble gas with atomic number 10.
Now, let’s break this down a little more:
Noble gases are found in Group 18 of the periodic table and are known for their lack of reactivity. This is because they have a full outer shell of electrons, making them very stable.
Magnesium, on the other hand, is a reactive metal. It readily loses its two outermost electrons to form ionic bonds with other elements, like oxygen to create magnesium oxide (MgO).
Think of it like this: magnesium wants to be like neon. They’re both in the same period of the periodic table (period 2), but neon has a full outer shell, while magnesium doesn’t. By losing its two electrons, magnesium takes on the same electronic configuration as neon and achieves stability.
What is the noble gas notation for Mg2+?
Let’s break down why this is significant. Noble gases, like Neon, are known for their stability. They have a full outer shell of electrons, which makes them very unreactive. When Magnesium loses two electrons to form the Mg²⁺ ion, it achieves this same stable configuration. This is why Mg²⁺ is so common in ionic compounds.
To write the noble gas notation for Mg²⁺, we use the symbol of the preceding noble gas, [Ne], to represent the filled inner shells. Since Mg²⁺ has the same electron configuration as Ne, its noble gas notation is simply [Ne].
The noble gas notation is a shorthand way of writing the electronic configuration. It makes it easier to see at a glance how many electrons are in the outermost shell of an atom or ion. And, in the case of ions like Mg²⁺, it helps us understand why they are so stable.
See more here: What Is Magnesium Noble Gas Notation? | Noble Gas Notation For Manganese
How to write a notation for manganese atom?
We can use the periodic table as a guide to help us write the electron configuration. The first step is to determine the principal quantum number (n) for each electron shell. The principal quantum number is simply the number of the electron shell. So, for manganese, the first electron shell has n = 1, the second electron shell has n = 2, and so on.
Next, we need to determine the number of electrons in each subshell. Each electron shell can have up to four subshells: s, p, d, and f. Each subshell can hold a specific number of electrons. The s subshell can hold a maximum of 2 electrons, the p subshell can hold a maximum of 6 electrons, the d subshell can hold a maximum of 10 electrons, and the f subshell can hold a maximum of 14 electrons.
We can use the periodic table to help us figure out how many electrons are in each subshell. The first two columns of the periodic table represent the s subshells, the next six columns represent the p subshells, the ten columns in the middle represent the d subshells, and the last fourteen columns represent the f subshells. To write the electron configuration for manganese, we start by filling the subshells in order of increasing energy.
For manganese, the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵
Let’s break down what this means:
– 1s²: The first shell (n=1) has 2 electrons in the s subshell.
– 2s²: The second shell (n=2) has 2 electrons in the s subshell.
– 2p⁶: The second shell (n=2) has 6 electrons in the p subshell.
– 3s²: The third shell (n=3) has 2 electrons in the s subshell.
– 3p⁶: The third shell (n=3) has 6 electrons in the p subshell.
– 4s²: The fourth shell (n=4) has 2 electrons in the s subshell.
– 3d⁵: The third shell (n=3) has 5 electrons in the d subshell.
This might seem like a lot of information, but it’s actually quite simple once you understand the basics. The periodic table is a great tool for helping you write electron configurations, so make sure you’re familiar with it. With a little practice, you’ll be able to write the electron configuration for any element!
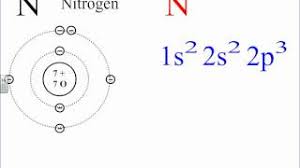
What is the electron configuration of manganese?
Manganese’s electron configuration tells us how its electrons are arranged in different energy levels and orbitals. It’s like a blueprint for how the atom is structured.
The electron configuration of manganese is [Ar] 3d⁵ 4s². This notation tells us several things:
[Ar] represents the electron configuration of the noble gas argon, which is the element that comes before manganese on the periodic table. This shortens the notation, as we don’t have to write out all the electrons for the first 18.3d⁵ indicates that there are five electrons in the 3d subshell. The 3d subshell is a higher energy level than the 4s subshell.
4s² means there are two electrons in the 4s subshell.
To understand this a bit deeper, let’s talk about orbitals.
Orbitals are regions of space around the nucleus where you’re most likely to find an electron. Each orbital can hold a maximum of two electrons. The 3d subshell has five orbitals, and the 4s subshell has one orbital.
Manganese’s five 3d electrons are distributed in these five orbitals, with one electron in each orbital. This arrangement follows Hund’s rule, which states that electrons will individually occupy orbitals within a subshell before pairing up in the same orbital.
This configuration reflects the stability of manganese. The half-filled 3d subshell makes manganese more stable. A filled or half-filled subshell is more stable than a partially filled subshell.
Let me know if you’d like to delve into other aspects of electron configuration or want to explore the properties of manganese in more detail!
How do you write a configuration for manganese ions?
First, we need to determine the electron configuration for a neutral manganese (Mn) atom. We can find the number of electrons in a manganese atom by looking at its atomic number on the periodic table – it’s 25!
Now, let’s write the electron configuration. We’ll fill the orbitals in order of increasing energy, following the Aufbau principle. Here’s the complete electron configuration for a neutral manganese atom:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵
Understanding the Electron Configuration
The configuration tells us how the 25 electrons are distributed across different energy levels and orbitals.
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the first three electron shells, which are completely filled.
4s² 3d⁵: This part is a bit tricky. Although the 4s subshell is filled before the 3d subshell, the 3d subshell is actually at a slightly higher energy level. So, the final five electrons go into the 3d subshell, giving manganese its unique properties.
Manganese Ions and Electron Configuration Changes
Now, let’s talk about manganese ions. Manganese can form various ions, like Mn²⁺, Mn³⁺, and Mn⁷⁺, each with a different electron configuration.
When manganese loses electrons to form a positive ion, it loses them from the highest energy level first. For example, to form Mn²⁺, manganese loses its two 4s electrons:
Mn²⁺: 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵
To form Mn³⁺, it loses one more electron, which comes from the 3d subshell:
Mn³⁺:1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁴
Remember, the number of electrons lost corresponds to the charge of the ion.
What is the chemical symbol for manganese?
The chemical symbol for manganese is Mn. It’s a transition metal, meaning it’s located in the d-block of the periodic table. Transition metals are known for their ability to form colorful compounds and have multiple oxidation states.
Manganese’s electron configuration is [Ar] 3d⁵ 4s². This means that manganese has a total of 25 electrons. The [Ar] represents the electron configuration of Argon, which is a noble gas. The 3d⁵ and 4s² represent the electrons in the 3d and 4s orbitals, respectively.
Manganese has a variety of oxidation states, the most common being +2, +3, +4, and +7. This means that manganese atoms can lose 2, 3, 4, or 7 electrons to form ions. The specific oxidation state of manganese in a compound depends on the other elements present and the chemical environment.
Let’s explore a little more about manganese’s oxidation states. Oxidation states describe the number of electrons an atom has gained or lost in a chemical reaction. For example, manganese in the +2 oxidation state has lost two electrons, while manganese in the +7 oxidation state has lost seven electrons. This variation in oxidation states is what gives manganese its diverse chemistry and makes it useful in a variety of applications.
The most common oxidation states of manganese are:
+2: Found in compounds like manganese(II) chloride (MnCl₂) and manganese(II) sulfate (MnSO₄). These compounds are often pale pink or white.
+3: Found in compounds like manganese(III) oxide (Mn₂O₃). This compound is a brown solid.
+4: Found in compounds like manganese dioxide (MnO₂). This compound is a black solid and is often used as a battery component.
+7: Found in compounds like potassium permanganate (KMnO₄). This compound is a strong oxidizing agent, often used as a disinfectant and in chemical reactions.
These oxidation states are important because they influence manganese’s chemical behavior and its applications. For example, manganese in the +7 oxidation state is a strong oxidizing agent, which means it can readily accept electrons from other substances. This property makes manganese compounds like potassium permanganate useful in a variety of applications, including disinfectants and chemical reactions.
So, next time you see Mn on a periodic table, remember this fascinating element with its diverse oxidation states and its important role in various applications.
See more new information: countrymusicstop.com
Noble Gas Notation For Manganese: A Step-By-Step Explanation
Understanding Noble Gas Notation
Before we get into the specifics of manganese, let’s understand the concept of noble gas notation. It’s a convenient way to represent the electronic configuration of elements, making it easier to grasp their chemical behavior.
Think of it like this: you have a really long address, but you can simplify it by using a well-known landmark. Noble gas notation is like using that landmark. We use the electronic configuration of the nearest preceding noble gas as a starting point and then add the remaining electrons.
Now, let’s look at noble gases themselves. They’re located in Group 18 of the periodic table and are known for their stability. They have a full outer shell of electrons, making them very unreactive. They’re like the “chill” kids at the back of the class.
Here’s how to write the noble gas notation for an element:
1. Identify the noble gas that comes before the element in the periodic table. For example, if we’re looking at manganese, the preceding noble gas is argon (Ar).
2. Write the symbol of the noble gas in square brackets. So, for manganese, we’ll start with [Ar].
3. Add the remaining electrons in the outer shells, following the usual rules of electronic configuration.
Applying Noble Gas Notation to Manganese
Now, let’s apply this knowledge to manganese (Mn). Its atomic number is 25, meaning it has 25 protons and 25 electrons.
1. Locate the nearest preceding noble gas:Argon (Ar) has an atomic number of 18, so it comes before manganese.
2. Write the noble gas in square brackets:[Ar]
3. Determine the remaining electrons:Manganese has 7 more electrons than argon (25 – 18 = 7).
Now, we need to figure out where these 7 electrons go. Let’s recall the rules for filling orbitals:
Aufbau principle: Electrons fill orbitals starting with the lowest energy level.
Hund’s rule: Each orbital in a subshell receives one electron before any orbital gets two.
Pauli exclusion principle: No two electrons in an atom can have the same set of four quantum numbers.
Following these rules, we can figure out the electronic configuration of manganese after argon. The 7 electrons are distributed as follows:
4s²: The 4s orbital gets filled first, holding two electrons.
3d⁵: The 3d orbital gets the remaining five electrons, one in each orbital according to Hund’s rule.
So, the noble gas notation for manganese is [Ar] 4s² 3d⁵.
Why is Noble Gas Notation Important?
You might be wondering why we go through all this trouble. Why not just write out the full electronic configuration?
Well, noble gas notation makes life easier! It’s a shorthand way to represent the electronic configuration, especially for elements with a lot of electrons. It also highlights the similarity in electron configuration between an element and the nearest preceding noble gas. This similarity helps us understand why elements behave the way they do, especially when it comes to chemical bonding.
Think of it like using abbreviations. We don’t always write out “National Aeronautics and Space Administration” – we simply use NASA. Similarly, using noble gas notation is a compact way to represent the electronic configuration.
FAQs
How is Noble Gas Notation Useful?
Noble gas notation is a compact and convenient way to represent the electronic configuration of an element. It helps us understand:
Chemical behavior: It highlights the number of valence electrons, which are the electrons involved in chemical bonding.
Reactivity: Elements with similar electronic configurations tend to have similar reactivity.
Trends in the periodic table: It helps us see patterns in the electronic configuration across the periodic table.
What is the Difference Between Electron Configuration and Noble Gas Notation?
Both electron configuration and noble gas notation represent the arrangement of electrons in an atom. However, they differ in how they present the information:
Electron configuration: Lists all the orbitals and their electron count in order of increasing energy levels.
Noble gas notation: Uses the configuration of the nearest preceding noble gas as a starting point, then lists the remaining electrons.
Why do we use Noble Gas Notation?
Noble gas notation offers several advantages over writing the full electron configuration:
Simplicity: It’s a shorter and more concise way to represent the electronic configuration, especially for larger atoms.
Clarity: It highlights the similarity between an element and the nearest preceding noble gas.
Understanding of trends: It helps us understand periodic trends in reactivity and chemical properties.
Can we use Noble Gas Notation for All Elements?
Yes, you can use noble gas notation for all elements. The only difference is the noble gas used as the starting point. For example, for elements in the second period (like lithium, beryllium, etc.), the preceding noble gas would be helium (He).
Understanding noble gas notation is a valuable tool for exploring the fascinating world of elements and their chemical properties. Remember, it’s all about finding the most convenient and understandable way to represent the arrangement of electrons in atoms!
How do you write the noble-gas electron configuration for
Explanation: …the nearest Noble gas is argon, Z=18 .. And so we do not have to specify the configuration of the first 18 electrons, because these approximate the configuration of argon.. Z = 18,1s22s22p63s23s6 electronic configuration of argon. For Socratic
Electron Configuration Chart of All Elements (Full Chart)
The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Periodic Table Guide
5.20: Noble Gas Configuration – Chemistry LibreTexts
This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called Chemistry LibreTexts
Electron Configuration for Mn, Mn2+, Mn3+ , and Mn4
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the number of YouTube
Electron Configuration for Manganese (Mn, Mn2+, Mn4+)
The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, the valence electrons of manganese are seven. Valenceelectrons.com
What is the Noble Gas notation (Electron configuration) for the …
Well what is #Z# for manganese….? Explanation: My Periodic Table tells me #Z=25# …and if there are 25 positive, nuclear charges, there are 25 electronic charges, Socratic
Manganese – Electron Configuration and Oxidation
For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. The electron configuration can be visualized as the core electrons, Periodic Table of Elements
Manganese – Element information, properties and uses
The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point The The Royal Society of Chemistry
2.4 Electron Configurations – Chemistry LibreTexts
All noble gases have their subshells filled and can be used them as a shorthand way of writing electron configurations for subsequent atoms. This method of writing configurations is called the noble gas notation, in Chemistry LibreTexts
Electron Configuration With Noble Gas Notation
Pseudo Noble Gas Electron Configurations
Noble Gas Notation – Ap Chemistry
Noble Gas Notation
Noble Gas Configuration
Link to this article: noble gas notation for manganese.
See more articles in the same category here: blog https://countrymusicstop.com/wiki
